Leaf Morphology and Pollen Viability of Blackgram [Vigna Mungo (L.) Hepper] Genotypes Under Heat Stress Condition
0 Views
C. HARITHA*, P. SANDHYA RANI, P. LATHA AND S. HEMALATHA
Department of Crop Physiology, S.V. Agricultural College, ANGRAU, Tirupati.
ABSTRACT
Laboratory experiment was conducted to screen fifty blackgram genotypes for high temperature tolerance using Temperature Induction Response (TIR) technique with nine tolerant blackgram genotypes viz., LBG 977, PU 1504, LBG 982, LBG 977, ABF 04, LBG 973, NRISRI, TBG 129, LBG 888 and one susceptible genotype TBG 125 were selected based on TIR for further screening them for high temperature stress tolerance under field condition. Sowings were carried in such a way that flowering of the genotypes coincided with the high temperatures. A wide variation was observed among the genotypes in their leaf characters such as leaf thickness, leaf pubescence, cuticle thickness and pollen viability percentage. The genotypes LBG 888 and TBG 129 were found to have higher leaf thickness, pubescence and pollen viability percentage, which denote their heat tolerance and ability to withstand higher temperature. Whereas, the susceptible genotype TBG 125 recorded lower values for leaf thickness pubescence and pollen viability percentage.
KEYWORDS:
Blackgram, Leaf thickness, Leaf pubescence, Pollen viability.
INTRODUCTION
Blackgram is a tropical leguminous plant belongs to the Asiatic Vigna species. Among grain legumes blackgram thrives better in all the seasons and can be grown as a sole crop, intercrop or as a fallow crop. It is an important pulse with high nutritive value and consists of high proteins, vitamins, amino acids and minerals thus; it is an important part in the dietary practices for large population in the world.
Constraints in blackgram production includes abiotic stresses, abrupt climatic changes, emergence of new insect-pests, diseases and deficiency of secondary & micronutrients in soils. (Ali and Gupta, 2012). Among pulses, blackgram accounts for 28 percent of world total grain legume production. It can be grown both summer and winter seasons (Sritharan et al., 2015).
Economic yield of blackgram mainly depends on physiological traits, such as ability to produce high biomass and partitioning of the photosynthates to reproductive organs. Biomass production depends on the extent of solar energy intercepted and its utilisation efficiency. In blackgram high temperature and drought are the most important constraint causing about 50 per cent of the yield loss (Anitha et al., 2015
Reproductive tissues are highly sensitive to heat stress, and a few degrees raise in temperature during flowering can lead to loss in the economic produce. Inside a flower ovules are more resistant to high temperature than pollen and anthers. (Sharma et al., 2016). Under high temperature (30°C), floret sterility has been correlated with diminished anther dehiscence, poor shedding of pollens, low pollen germination percentage , decreased pollen tube elongation and reduced in vivo pollen germination (Fahad et al., 2015). Plants use different mechanisms to control leaf temperature, like changing the leaf traits like hairiness, colour and thickness. (Monteiro et al., 2016). Keeping these in view, a field experiment was conducted to evaluate leaf morphological traits and pollen viability of blackgram genotypes under high temperature condition.
MATERIAL AND METHODS
The experiment was laid out in a randomized block design with 3 replications and 10 blackgram genotypes viz., LBG 977, PU 1504, LBG 982, LBG 977, ABF 04,
LBG 973, NRISRI, TBG 129, LBG 888 and TBG 125
(susceptible genotype) were sown in the wet land farm of S.V. Agricultural College, Tirupati, ANGRAU, during the 1st fortnight of February 2020.
Leaf thickness (mm)
Leaf thickness was measured with the Verniercalliper at random locations on leaf, excluding the mid rib and represented in millimeters (mm)
Leaf pubescence (No. of trichomes per sq.cm)
Leaf pubescence was measured by using stereomicroscope (40X). The leaflet was cut into bits of 1 cm2 and number of trichomes present on the upper and lower surface was counted under stereo zoom trinocular microscope and expressed as number of trichomes per square centimeter of leaf area.
Pollen viability test
The pollen grains from anthers of randomly selected flowers were collected and taken on cavity slides and stained with iodine-potassium iodide solution (0.44 g Iodine + 20.8 g potassium iodide in 500 ml of 70% alcohol). The viable pollen turns immediately to dark blue and non-viable ones remained as light yellow. The number of viable and non-viable ones were counted using OLUMPUS SZ61 microscope. The viability percentage was calculated from the mean of three microscopic field counts for each genotype (Jensen, 1962)
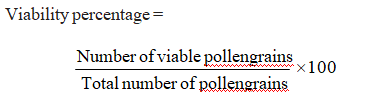
Cuticle thickness (µm)
Fresh leaf samples were collected and fixed in cold FAA (Formalin – acetic acid- alcohol) and dehydrated in a tetra- butyl alcohol series (Berlyn and Miksche, 1976) and embedded in paraffin. Thin sections were made and mounted on slides and stained for 1hr with Sudan III in ethylene glycol (Jensen, 1962). Thickness of cuticle was measured with the help of calibrated ocular and stage micrometer at 400X under oil immersion. (Delucia and Berlyn, 1983). For calibration of stage micrometer, the number of ocular division coinciding with the stage division was found out. After calibration, stage micrometer is removed and the sample is placed on the stage slide and focused. The number of ocular division occupied by leaf sample was counted. Multiplying the number of division with calibration factor gives thickness of cuticle. Three such readings were taken and average is determined.
RESULTS AND DISCUSSION
Leaf thickness (mm)
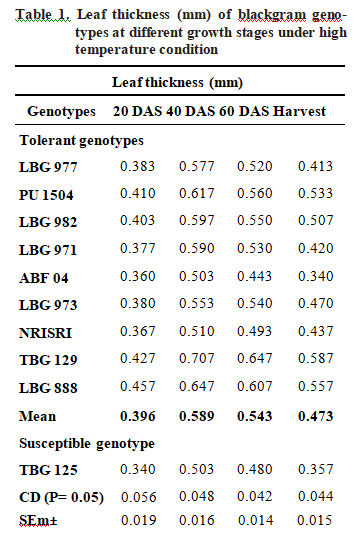
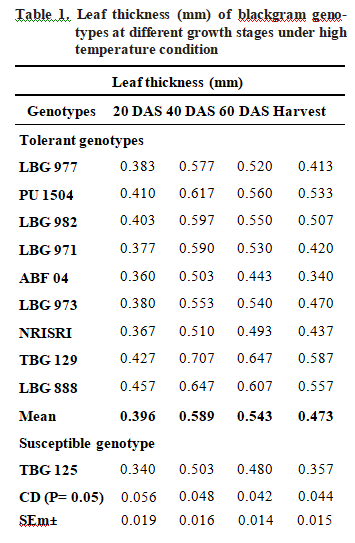
Leaf thickness was measured at various stages of crop growth i e. at 20, 40, 60 days after sowing (DAS) and at harvest presented in Table 1. All genotypes showed reduction in leaf thickness from 40 DAS onwards. Among the genotypes TBG 129 showed highest value for thickness during entire crop growth stages. At harvesting stage TBG 129, LBG 888 and PU 1504 showed highest value of leaf thickness as 0.587 mm, 0.557 mm and 0.533 mm respectively. Thermo sensitive genotype TBG 125 showed lower leaf thickness during all growth stages. The possible reason for decrease in the leaf thickness at later stages of crop might be due to accelerated leaf senescence at high temperature condition. The results from this study implies that genotypes such as TBG 129, LBG 888 and PU 1504 showed high leaf thickness compared to susceptible genotype TBG 125. These findings are in conformity with Salem-fnayou et al. (2011), Groom et al. (2004) and Givnish (1978).
Leaf pubescence (No. of trichomes sq cm-1)
All genotypes showed increase in the number of trichomes sq cm-1 during the entire crop growth period. The rate of increase was less during later stages of crop growth. i.e at 60 DAS. Among the tolerant and susceptible genotypes, mean number of trichomes per sq. cm of tolerant genotypes was higher at all growth stages i.e. at 20 DAS (11.41), at 40 DAS (19.44) and at 60 DAS (20.52)
compared to susceptible genotype TBG 125. During the flowering time LBG 888 showed highest number of trichomes per sq. cm (25), followed by TBG 129 (23), and PU 1504 (21.67) and was lowest for ABF 04 (14.67). TBG 129 showed highest value (24.67) followed by TBG 129 and PU 1504. ABF 04 and TBG 125 showed lowest value i.e. 15 and 15.67 respectively. The results from the current study displays that trichomes present on the leaf surface minimize the rate of transpiration by means of high boundary layer resistance thus increase the water use efficiency of the crop further pubescence reduces leaf absorbance ensuring in reduced heat load and as a consequence lower leaf temperature, much lower than air temperature. As a result, leaf temperatures are near the temperature optimum for photosynthesis. This is why tolerant genotypes showed more number of trichomes than susceptible one. These present findings are in conformity with Roy and Basu (2009) and Monteiro et al. (2016).
Cuticle thickness (µm)
Cuticle thickness (μm) measured at various phases of crop growth was presented in Table 2. Significant difference was there between genotypes for thickness of cuticle throughout the crop growth period. Results showed that cuticle thickness gradually increased upto 60 DAS in all genotypes and later decreased. Genotype PU 1504 showed highest value of cuticle thickness during all phases of crop growth and lowest value was showed by thermo sensitive genotype TBG 125. Reduction in cuticle thickness at harvest stage might be because the cuticle deposition is persistent until the leaf reaches morphological maturity after which no further deposition occurs. High cuticular thickness of thermo tolerant genotypes linked to water loss prevention under dry summer conditions. These findings are in accordance with England and Attiwill, (2011), Shepherd et al. (2006).
Pollen viability percentage (%)
Heat tolerant and sensitive genotypes varied in pollen viability when exposed to high temperature. Result showed that viability percentage varied from 60.77 to
86.77. Lowest pollen viability was showed by susceptible genotype (60.77) and highest value was showed by LBG 888 (86.77) followed by TBG 129 (81.64) (Fig. 1.)
Poor pollen viability might be the product of under nourished pollen due to stress during growth. Tapetal layers in anthers, which contribute nutrients to pollen production, are the subject of thermal stress as recorded in cowpea (Ahmed et al. 1992), chickpea (Kumar et al. 2010) and mungbean (Porch and Jahn, 2011). Result revealed that viability percentage was lowest for thermo sensitive genotype TBG 125. This is because the function of pollen, stigma, and ovule in heat tolerant genotypes was held significantly higher than sensitive one. Those findings are consistent with previous studies in chickpea Kumar et al. (2013).
From the investigation the following conclusions were drawn, among the genotypes screened TBG 129 showed highest leaf thickness and susceptible genotype TBG 125 with lowest leaf thickness. Reduction in pollen viability was more in sensitive genotype TBG 125 and LBG 888 recorded highest pollen viability percentage. High summer temperature during flowering time leads to reduction in pollen viability percentage in susceptible genotype TBG 125. Among the morphological characters studied such as leaf thickness, leaf pubescence and cuticle thickness genotype LBG 888 and TBG 129 showed highest value compared to all other genotypes which made them more tolerant to temperature stress.
LITERATURE CITED
Ahmed, F.E., Hall, A.E and Demason, D.A. 1992. Heat injury during floral development in cowpea (vigna unguiculata, fabaceae). American Journal of Botany. 79(7): 784-791.
Ali, M and Gupta, S. 2012. Carrying capacity of Indian agriculture. Current Science. 102(6): 874-881.
Anitha, Y., Vanaja, M and Kumar, G.V. 2015. Identification of Attributes Contributing to High Temperature Tolerance in Blackgram (Vigna mungo
L. Hepper) Genotypes. International Journal of Science and Research. 5(11): 1021-1024.
Berlyn, G.P and Miksche, J.P. 1976. Botanical microtechnique and cytochemistry. Iowa State University Press.
Delucia, E.H and Berlyn, G.P. 1983. The effect of increasing elevation on leaf cuticle thickness and cuticular transpiration in balsam fir. Canadian Journal of Botany. 62: 2423-2431.
England, J.R and Attiwill, P.M. 2011. Changes in stomatal frequency, stomatal conductance and cuticle thickness during leaf expansion in the broad leaved ever green species, Eucalyptus Regnans. Trees. 25: 987-996.
Fahad, S., Hussain, S., Saud, S., Tanveer, M., Bajwa, A.A and Hassan, S. 2015. A biochar application protects rice pollen from high-temperature stress. Plant Physiology and Biochemestry. 96: 281–287.
Givnish, T.J. 1978. Ecological aspects of plant morphology: leaf form in relation to environment. Acta Biotheoretica. 27: 83-142
Groom, P.K., Lamont, B.B., Leighten, S and Burrows, C. 2004. Heat damage in sclerophylls is influenced by their leaf properties and plant environment. Ecoscience. 11(1): 94-101.
Jensen, W.A. 1962. Botanical Histochemistry, Freeman & Co. Sanfransisco.
Kumar, S., Nayyar, H., Bhanwara,R.K and Upadhyaya,
H.D. 2010. Chilling stress effects on reproductive biology of chickpea. Journal of Semi Arid Tropic Research. 8: 1-14.
Kumar, S., Thakur, P., Kaushal, N., Malik, J.A., Gaur, P and Nayyar, H. 2013. Effect of varying high temperature during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Agronomy and Soil Science. 59: 823-843.
Monteiro, M., Blanusa, T., Verhoef, A., Hadley, P and Cameron, R. 2016. Relative importance of transpiration rate and leaf morphological traitfor the regulation of leaf temperature. Australian Journal of Botany, 64(1): 32-44.
Porch, T.G and Jahn, M. 2001. Effects of high-temperature stress on microsporogenesis in heat-sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant, Cell and Environment. 24: 723-731.
Roy, B and Basu, A.K. 2009. Abiotic stress tolerance in crop plants. New India Publishing Agency.
Salem-fnayou, A.B., Bouamama, B., Ghorbel, A and Mliki, A. 2011. Investigations on the leaf anatomy and ultrastructure of grapevine under heat stress. Microscopy Research and Technique. 74: 756-762.
Sharma, L., Priya, M., Bindumadhava, H., Nair, R.M and Nayyar, H. 2016. Influence of high temperature stress on growth, phenology and yield performance of mungbean under managed growth conditions. Scientia Horticulturae. 213: 379-391.
Shepherd, T and Grffiths, D. 2006. The effects of stress on plant cuticular waxes. New Phytology. 171: 469- 499.
Sritharan, N., Rajavel, M and Senthilkumar, R. 2015. Physiological approaches: Yield improvement in blackgram. Legume Research. 38(1): 91-95.
- Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
- Brand Preference of Farmers for Maize Seed
- Issues That Consumer Experience Towards Online Food Delivery (Ofd) Services in Tirupati City
- Influence of High Density Planting on Yield Parameters of Super Early and Mid Early Varieties of Redgram (Cajanus Cajan (L.) Millsp.)
- Influence of Iron, Zinc and Supplemental N P K on Yield and Yield Attributes of Dry Direct Sown Rice
- Effect of Soil and Foliar Application of Nutrients on the Performance of Bold Seeded Groundnut (Arachis Hypogaea L.)

