A METHOD FOR CONTINUOUS REARING OF Maruca vitrata (GEYER) ON NATURAL DIET
0 Views
L.P. VENKATA REDDY*, K.V. HARIPRASAD, T. MURALI KRISHNA, P. LATHA AND S. KHAYAUM AHMMEED
Department of Entomology, S.V. Agricultural College, Tirupati, A.P. 517 502
Laboratory rearing and continuous supply of insects is a pre-requisite for undertaking studies on various IPM tools such as germplasm evaluation as a part of host plant resistance studies (Sharma et al., 2005), Insecticide/ biopesticide evaluation (Surekha Devi et al., 2011), studies on tri trophic interactions involving host plant, insect and natural enemy (Routray and Hariprasad, 2016), studies on transgenic crops., etc.,
The spotted pod borer Maruca vitrata (Geyer) is one of the most important insect pest of grain legumes in tropical and sub-tropical countries including India. It is a serious pest of pigeonpea (Sharma, 1999; Randhawa, 2013); field bean, cowpea, mung bean and urd bean (Chandrayudu et al., 2006; Halder and Srinivasan, 2007).
While evaluating greengram and blackgram varieties for plant resistance to M. vitrata, at the Dept. of Entomology, S V Agricultural College, Tirupati, we have noticed cannibalism of mature Maruca larvae (Fig. 1) not only among the larval instars (Soundararajan and Chitra, 2014) but also on pupae, which has not been reported earlier. The per cent cannibalism on larvae was 30 to 50 (Table 1).
To overcome this cannibalism, we have established a method for continuous rearing of M. vitrata on natural diet as detailed below:.
1st and 2nd instars:
Cannibalism was not observed in these instars. 1st instars immediately after hatching from eggs were transferred in a batch of 2-3 and were placed in multi-cell wellplates with redgram flowers as food material. The larval duration of both 1st and 2nd instar lasted for 60 to 72 hours (2.5 to 3 days). Fresh flowers were provided as and when required without disturbing the feeding activity and larvae.
Cannibalism was observed from 3rd instar and continued up to 5th instar.
3rd and 4th instar:
The instar duration was around 90 – 100 hours for both the instars. As the cannibalism started from 3rd instar onwards, the larvae were carefully separated with a camel brush and reared individually in multi-cell well plates with pods of redgram or fieldbean as food material.
The larvae made webbings of the food material and fed within the webbings. The food material was replenished as and when needed during which the multicell well plates were cleaned with absolute alcohol to prevent fungal contamination. The same procedure was followed for rearing 4th instar larvae where the instar duration was around 90- 100 hrs.
5th instar and pre pupal period:
The duration of 5th instar was 65 to 72 hrs. The larvae in 5th instar were generally light bluish green in color. The pre pupal period lasted for about 20-24 hrs. Larvae during pre- pupal period were not disturbed to avoid any chances of mortality during handling.
Pupae:
Pupation was observed within webbing. The freshly formed pupa were light brownish in color with green tinge and after 12 hrs the color changed to complete brown. Once the pupae turn brownish, they were separated and placed in oviposition cages for adult emergence and egg laying. The pupal period was around 96-120 hrs.
Oviposition:
The pupae were kept in a Petri plate of 9 cm diameter and placed in the oviposition cage with redgram flowers as substrate. Diluted honey (5%) was provided as food material for the adults. The flowers were changed every day and observed for the eggs deposited.
Rearing of early instars i.e. 1st and 2nd with flower buds of redgram or fieldbean, followed by individual rearing of larvae i.e. 3rd, 4th, and 5th larvae and segregation of larvae and pupae was suggested as the best method for continuous rearing of M. vitrata larvae successfully without cannibalism (Fig. 2).
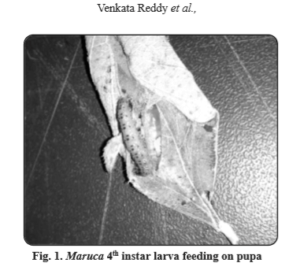
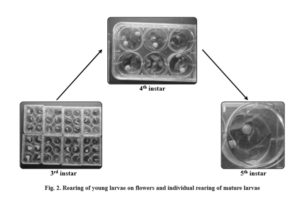
A method of continuous rearing of Maruca vitrata on natural diet
LITERATURE CITED
Chandrayudu, E., Srinivasan, S and Rao, N.V. 2006. Incidence of Maruca vitrata in grain legumes in relation to plant characters. Annals of Plant Protection Sciences. 14(2): 465-466.
Halder, J and Srinivasan, S. 2007. Biochemical basis of resistance against Maruca vitrata (Geyer) in Urd bean. Annals of Plant Protection Sciences. 15(2): 287-290.
Sharma, H.C., Pampapathy, G and Kumar, R. 2005. Standardization of cage technique to screen chickpeas for resistance to Helicoverpa armigera (Lepidoptera: Noctuidae) under greenhouse and field conditions. Journal of Economic Entomology. 98: 210-216.
Sharma, H.C., Saxena, K.B and Bhagwat, V.R. 1999. The legume pod borer, Maruca vitrata: bionomics and management. Information Bulletin, 55. ICRISAT, Patancheru, Andhra Pradesh.
Soundararajan, R.P. and Chitra, N. 2014. Larval cannibalism in spotted pod borer, Maruca vitrata (Geyer), Insect Environment, 20(1), April-June 2014. 17.
Surekha, Devi V., Sharma, H.C. and Arjun Rao, P. 2011. Interaction between host plant resistance and biological activity of Bacillus thuringiensis in managing the pod borer Helicoverapa armigera in chickpea. Crop Protection. 30: 962-969.
Randhawa, H.S. 2013. Evaluation of pigeonpea genotypes for their resistance against pod borer, Maruca vitrata (Geyer) under natural conditions. Trends in Biosciences. 6(5): 562-563.
Routray, S and Hariprasad, K.V. 2016. Tri-Trophic interaction involving host plants, black legume aphid, Aphis craccivora (Hemiptera: Aphididae) and the predator, Cheilomenes sexmaculata (Coleoptera: Coccinellidae). European Journal of Entomology. 113: 551–557.
- Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
- Brand Preference of Farmers for Maize Seed
- Issues That Consumer Experience Towards Online Food Delivery (Ofd) Services in Tirupati City
- Influence of High Density Planting on Yield Parameters of Super Early and Mid Early Varieties of Redgram (Cajanus Cajan (L.) Millsp.)
- Influence of Iron, Zinc and Supplemental N P K on Yield and Yield Attributes of Dry Direct Sown Rice
- Effect of Soil and Foliar Application of Nutrients on the Performance of Bold Seeded Groundnut (Arachis Hypogaea L.)

