STUDIES ON THE ROLE OF SYNERGISTS FOR COUNTERING DEVELOPMENT OF INSECTICIDE RESISTANCE IN LARVAL POPULATION Maruca vitrata (Geyer) FROM MAJOR BLACKGRAM GROWING AREAS OF ANDHRA PRADESH
0 Views
T. NARESH*, P. RAJASEKHAR, K.V. HARI PRASAD, V.L.N REDDY, B. RAVINDRA REDDY AND N.C. VENKATESWARLU
Department of Entomology, S.V. Agricultural College, ANGRAU, Tirupati – 517502, Andhra Pradesh
ABSTRACT
Role of synergist for countering the insecticide resistance mechanism was studied in both baseline and field populations during rabi 2018-19 at Insectary, Department of Entomology, S.V. Agricultural College, Tirupati. Higher per cent mortality was observed in larvae treated with insecticide + synergist against insecticide alone. Mean per cent mortality of insecticide + synergist and insecticide alone significantly differed. The highest percent mortality was observed with chlorantraniliprole alone and along with synergist followed by spinosad, thiodicarb, dichlorvos and chlorpyriphos alone and along with synergist respectively. In baseline population, the highest percent mortality was observed in insecticide and insecticide along with synergist followed by Prakasam, Nellore, Chittoor, Kurnool and Guntur districts larval population.
KEYWORDS:
Maruca vitrata, chlorantraniliprole, spinosad, thiodicarb, dichlorvos, chlorpyriphos, PBO (Pyperonyl butoxide), sesamin.
INTRODUCTION
India is the world’s largest producer of blackgram contributing 28 per cent to the global pulse basket from an area of about 37 per cent, as well as largest consumer. Blackgram is the 3rd important crop and it was cultivated over an area of 5.44 million hectares (kharif + rabi) and recorded a production of 3.56 million tonnes at a productivity level of 655 kg ha-1. More than 90 per cent of urdbean production comes from nine states viz., Madhya Pradesh, Rajasthan, Uttar Pradesh, Andhra Pradesh, Tamil Nadu, Maharashtra, Jharkhand, Gujarat and West Bengal. In Andhra Pradesh blackgram is cultivated in an area of 3.81 lakh ha and with a total production of 3.13 lakh tonnes with productivity of 946 kg ha-1. Andhra Pradesh contributes 7.57 per cent and 9.53 per cent in area and production of blackgram in India (Indiastat, 2018).Blackgram contains 24 per cent protein, 3.2 per cent minerals, 59.6 per cent carbohydrate, amino acids and vitamins. It also contains 154 mg calcium, 9.1 mg iron and 38 mg â-carotene per 100g of split daul (Nene, 2006).
Insect pests are serious limiting factors in pulse production leading to reduced productivity. Blackgram is attacked by 40 to 60 insect species at different stages of the crop growth (Khazuria et al., 2015). On an average, 2.5 to 3.0 million tonnes of pulses are lost annually due to pest problems in India(Ramanujan, 2004). The yield loses on urd bean due to insect pests Maruca vitrata (Geyer), Spodoptera litura (Fabricius), thrips and pod bugs etc., at various stages of the crop growth accounts 30 to 54.3 per cent in India (Dhuri and Singh, 1983). Though an array of pests attacks this crop, the major loss is inflicted by the pod borer, M. vitrata the larvae of which damage both vegetative and reproductive stages of the crop (Ganapathy, 2010). It is an important pest affecting the grain legumes in tropics and subtropics and is reported to feed on 39 host plants (Manjunath and Mallapur, 2015). At present, farmers rely mainly on synthetic chemical insecticides to overcome the problem of M. vitrata. Indiscriminate use of insecticide also leads to detrimental effect on natural enemies, environmental and potential health hazards for people working in the field.
Synergists are among the most straight forward tools for overcoming metabolic resistance because they can directly inhibit the resistance mechanism itself. Since the first demonstration of insecticide synergism over 79 years ago (Eagleson, 1940), their effective application against agricultural pests has offered tremendous promise but achieved little utility. This is partly because of difficulties in their use and partly because there was lack of basic understanding of insect detoxification systems.
MATERIAL AND METHODS
Procedure for Bioassay
Each treatment consisted of ten larvae with four replications. Just before the insecticidal treatment, third instar larvae of M. vitrata were transferred carefully with camel brush in to clean and dry multi cavity cell trays at the rate of one larvae for one cell and covered with lid. The larvae were starved for two hours before treatment. For conducting bioassay studies flower buds dip bioassay method was used (Elzen et al., 1992; Sreelakshmi et al., 2015). 10 ml of quantity of five concentrations for each insecticide were prepared. Flower buds collected from the field were dipped in test insecticidal solutions for ten seconds and later shade dried. These flowers were placed in 9 cm diameter petriplate lined with filter paper; larvae were placed on these flowers and were allowed to feed on treated flowers. Control treatment was done with water alone.
Amount of insecticide required for preparation of required per cent concentration of insecticide solution was calculated by using below formula

Data recorded
Larval mortality was taken 24 hour, 48 hours and 72 hours after treatment. The larvae which were moribund and did not show any movement were also considered as dead.
Statistical analysis
Data on mortality was subjected to Abbott’s formula (Abbotts, 1925) for calculating corrected mortality percentage. LC50 values were determined by probit analysis (Finney, 1971) using Statistical Packages for Social Sciences (IBM SPSS Statistics 20) software.

After calculating the LC50 value 24 HAT calculate the amount of a.i present in that concentration and mixing the 1:10 ratios (Lorini and Galley, 2000) of insecticide and synergist concentration, respectively. Synergists viz., pyperonyl butoxide (PBO) and sesamin were used along with the test insecticides (at their respective LC50concentrations obtained from earlier bioassay experiment) against resistance larval population of M. vitrata,to determine the efficiency of synergists in enhancing the insecticide toxicity in the resistant larval population of M. vitrata. The insecticides viz., chlorpyriphos, thiodicarb, spinosad, chlorantraniliprole in combination with PBO and dichlorvos in combination with sesamin in the ratios of 1:10 were evaluated by flowers dip method of bioassay insecticides and synergists were diluted with acetone to the required doses i.e., 10 times more than the test doses of the insecticides (Table.1). The insecticides and synergists doses were mixed to get the insecticide synergist mixtures in 1:10 and test insect population were exposed till the mortality in the range of 5-90 per cent was recorded. Mortality was recorded 12 hours and 24 hours after treatment. Per cent mortality was calculated by using below formula
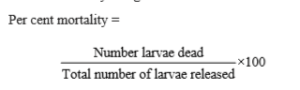
RESULTS AND DISCUSSION
The per cent larval mortality (12 HAT) in baseline population of M. vitrata was significantly higher when treated with chlorantraniliprole 67.78 per cent (significantly differs from other insecticide) followed by spinosad 61.62 per cent, thiodicarb 58.58 per cent (on par with each other), dichlorvos 52.53 per cent and chlorpyriphos 47.48 per cent (significantly differs from other insecticide) (Table 2).
The per cent larval mortality (24 HAT) in Baseline population of M. vitrata was significantly higher when treated with chlorantraniliprole 91.11 per cent followed by spinosad 88.89 per cent, thiodicarb 85.56 per cent, dichlorvos 83.33 per cent and chlorpyriphos 81.11 per cent (on par with each other) (Table 2).
There was significantly higher per cent mean larval mortality observed, when the larvae were treated with insecticide along with synergist (67.88%) as against
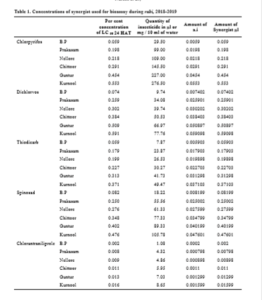
insecticide alone (47.27%) in 12 HAT and insecticide along with synergist (100%) as against insecticide alone (72.00%) in 24 HAT.
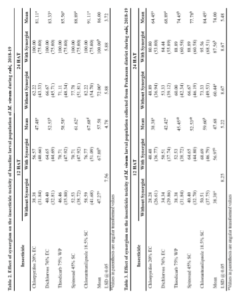
The per cent larval mortality (12 HAT) in Prakasam district population of M. vitrata was significantly higher when treated with chlorantraniliprole 59.60 per cent (significantly differs from other insecticides) followed by spinosad 52.53 per cent, thiodicarb 45.45 per cent (on par with each other), dichlorvos 42.42 per cent and chlorpyriphos 38.38 per cent (on par with each other) (Table 3).
The per cent larval mortality (24 HAT) in Prakasam district population of M. vitrata was significantly higher when treated with chlorantraniliprole 84.45 per cent (significantly differs from other insecticide) followed by spinosad 77.78 per, thiodicarb 74.45 per cent (on par with each other), dichlorvos 68.89 per cent and chlorpyriphos 64.45 per cent (on par with each other) (Table 3).
There was significantly higher per cent mean larval mortality observed, when the larvae were treated with insecticide along with synergist (56.97%) as against insecticide alone (38.88%) in 12 HAT and insecticide along with synergist (87.56%) as against insecticide alone (60.44%) in 24 HAT.
The per cent larval mortality (12 HAT) in Nellore district population of M. vitrata was significantly higher when treated with chlorantraniliprole 55.56 per cent (significantly differs from other insecticide) followed by spinosad 49.50 per cent (significantly differs from other insecticide), thiodicarb 43.44 per cent (significantly differs from other insecticide), dichlorvos 37.37 per cent and chlorpyriphos 34.34 per cent (on par with each other) (Table 4).
The per cent larval mortality (24 HAT) in Nellore district population of M. vitrata was significantly higher when treated with chlorantraniliprole 82.22 per cent (significantly differs from other insecticide) followed by spinosad 74.45 per, thiodicarb 71.11 per cent (on par with each other), dichlorvos 66.67 per cent and chlorpyriphos 62.22 per cent (on par with each other) (Table 4).
There was significantly higher per cent mean larval mortality observed, when the larvae were treated with insecticide along with synergist (52.93%) as against insecticide alone (35.15%) in 12 HAT and insecticide along with synergist (86.22%) as against insecticide alone (56.44%) in 24 HAT.
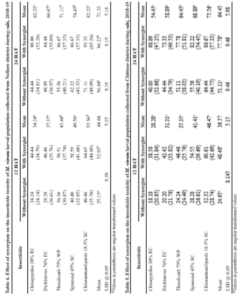
The per cent larval mortality (12 HAT) in Chittoor district population of M. vitrata was significantly higher when treated with chlorantraniliprole 46.47 per cent followed by spinosad 41.41 per cent, thiodicarb 35.35 per cent, dichlorvos 31.31 per cent and chlorpyriphos 28.28 per cent (on par with each other) (Table 5).
The per cent larval mortality (24 HAT) in Chittoor district population of M. vitrata was significantly higher when treated with chlorantraniliprole 75.56 per cent (significantly differs from other insecticide) followed by spinosad 68.89 per, thiodicarb 64.45 per cent (on par with each other), dichlorvos 58.89 per cent and chlorpyriphos 54.45 per cent (on par with each other) (Table 5).
There was significantly higher per cent mean larval mortality observed, when the larvae were treated with insecticide along with synergist (48.48%) as against insecticide alone (24.65%) in 12 HAT and insecticide along with synergist (77.78%) as against insecticide alone (51.11%) in 24 HAT.
The per cent larval mortality (12 HAT) in Kurnool district population of M. vitrata was significantly higher when treated with chlorantraniliprole 42.42 per cent (significantly differs from other insecticide) followed by spinosad 36.36 per cent (significantly differs from other insecticide), thiodicarb 31.31 per cent, dichlorvos 27.27 per cent and chlorpyriphos 25.25 per cent (on par with each other) (Table 6).
The per cent larval mortality (24 HAT) in Kurnool district population of M. vitrata was significantly higher when treated with chlorantraniliprole 70.00 per cent followed by spinosad 65.56 per (significantly differs from other insecticide), thiodicarb 57.78 per cent (on par with each other), dichlorvos 53.33 per cent and chlorpyriphos 48.89 per cent (on par with each other) (Table 6).
There was significantly higher per cent mean larval mortality observed, when the larvae were treated with insecticide along with synergist (42.83%) as against insecticide alone (22.22%) in 12 HAT and insecticide along with synergist (71.56%) as against insecticide alone (46.67%) in 24 HAT. The per cent larval mortality (12 HAT) in Guntur district population of M. vitrata was significantly higher when treated with chlorantraniliprole 37.37 per cent (significantly differs from other insecticide) followed by spinosad 31.31 per cent (significantly differs from other
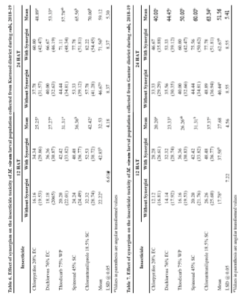
insecticide), thiodicarb 26.26 per cent, dichlorvos 23.33 per cent and chlorpyriphos 20.20 per cent (on par with each other) (Table 7).
The per cent larval mortality (24 HAT) in Guntur district population of M. vitrata was significantly higher when treated with chlorantraniliprole 63.34 per cent followed by spinosad 60.00 per cent, thiodicarb 50.00 per cent (on par with each other), dichlorvos 44.45 per cent and chlorpyriphos 40.00 per cent (on par with each other) (Table 7).
There was significantly higher per cent mean larval mortality observed, when the larvae were treated with insecticide along with synergist (37.58%) as against insecticide alone (17.78%) in 12 HAT and insecticide along with synergist (62.67%) as against insecticide alone (40.44%) in 24 HAT.
In present investigations more per cent larval mortality was observed in insecticide + synergist treated larval population when compared with insecticide alone treated population. These findings were supported by Yu (1983) who revealed that piperonyl butoxide, the wellknown inhibitor of microsomal oxidases, enhanced the toxicity of OP compound insecticides in M. vitrata and also Armes et al. (1997) reported that treatment with the metabolic inhibitor, piperonyl butoxide, resulted in complete suppression of cypermethrin resistance (2- to 121-fold synergism), indicating that enhanced detoxification by microsomal P450-dependent monooxygenases was probably the major mechanism of pyrethroid resistance. Wang et al. (2009) reported that after inhibitors were used, spinosad resistance could be partially suppressed by piperonylbutoxide (PBO) and triphenylphosphate (TPP), but not by diethyl maleate (DEM) in Helicoverpaarmigera. Baoet al. (2014) reported that the synergist, piperonyl butoxide, respectively caused 1.1-fold, 5.8-fold and 9.0-fold decreases in the resistance ratios of the OK, TS1, and TS5 strains of Thripspalmi against spinosad. The maximum per cent mortality reached 48 HAT because of these synergists were inhibit the mechanism of detoxification enzymes in larval population of M. vitrata. Present results were also supported by results of Wangaet al. (2018) who conducted chlorpyriphos synergist (PBO) experiment and indicated that the Lab-HN strain and fieldcollected population of S. litura from Shuangliu (China) in 2016 (SL16 population) showed that, the highest synergism ratios (SR) of 34.4 and 73.3 fold respectively.
The results of Bingham et al. (2008) were also in agreement with present findings, stated that PBO reduced the pirimicarb resistance factor in a clone of Myzuspersicae (Sulzer) from >19000 to 100 fold and in Aphis gossypii (Glover) from >48000 to 30 fold. Similar results were obtained for a strain of Bemisiatabaci Gennadius. Synergism was also observed in laboratory susceptible insects, suggesting that, even when detoxification is not enhanced, there is degradation of insecticides by the background enzymes. Use of an analogue of PBO, which inhibits esterases but has reduced potency against microsomal oxidases, suggests that acetamiprid resistance in whiteflies is largely oxidase based. Present results are also supported by Sohail and Irfanullah (2004) who used sesame oil, as the synergist. The insecticide was mixed with sesame oil in 1:1 and 1:2 ratios to obtain LC50 of the insecticide + synergist combination. House fly was allowed to feed on the insecticide coated sugar for 48 hr. The mortality data was observed after 1, 2, 4, 6, 8, 12, 24 and 48 hours. The results showed that dichlorvos was the most effective with LC50 value, 122 ppm and with the synergist combination.
Role of synergist for countering the insecticide resistance mechanism in all populations were studied and the highest percent mortality was observed in larval population treated with chlorantraniliprole alone and along with synergist followed by spinosad, thiodicarb, dichlorvos and chlorpyriphos alone and along with synergist respectively. Chlorantranilip -role in combination with piperonyl butoxide gave maximum mortality in all population of M. vitrata 12 and 24 HAT. Maximum per cent morality was observed in baseline population followed by Prakasam, Nellore, Chittoor, Kurnool and Guntur during rabi 2018-19.
LITERATURE CITED
Abbotts, W.S. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 18: 265-267.
Armes, N.J., Wightman, J.A., Jadhav, D.R and Rao, G.V.R. 1997.Status of insecticide resistance in Spodoptera litura in Andhra Pradesh, India. Journal of Pesticide Science. 50: 240–248.
Bao, W.X., Narai, Y., Nakano, A., Kaneda, T., Murai, T and Sonoda, S. 2014. Spinosad resistance of melon thrips, Thrips palmi, is conferred by G275E mutation in a6 subunit of nicotinic acetylcholine receptor and cytochrome P450 detoxification. Pesticide Biochemistry and Physiology. 1-5.
Bingham, G., Gunning, R.V., Delogu, V.G., Borzatta, A., Field, L.M and Moores, G.D. 2008. Temporal synergism can enhance carbamate and neonicotinoid insecticidal activity against resistant crop pests. Pest Management Science. 64: 81–85.
Dhuri, A.V and Singh K.M. 1983.Pest complex and succession of insect pests in black gram. Indian Journal of Entomology. 45: 396-401.
Eagleson, C. 1940. U. S. Patent 2, 202, 145.
Elzen, G.W., Leonard, B.R., Graves, J.B., Burris, E and Micinski, S. 1992. Resistance to pyrethroids, carbamates, and organophosphate insecticides in field populations of tobacco budworm (Lepidoptera: Noctuidae) in 1990. Journal of Economic Entomology. 85: 20642072.
Finney, D.J. 1971. Probit Analysis. Cambridge University Press, Cambridge, UK.
Ganapathy, N. 2010. Spotted pod borer, Maruca vitrata (Geyer) in Legumes: Ecology and Management. Madras Agricultural Journal. 97(9): 199-211.
Indiastat.com.2018.https://www.indiastat.com/ agriculture-data/2/agricul-tural production/22 5/urdblack-gram/19573/stats.aspx. State-wise area, production and productivity of blackgram in India
Khazuria, S., Rai, A.K., Kumar, K.L.R and Jadav, S. 2015. Evaluation of integrated pest management module against sucking pests of black gram under semiarid conditions. Insect Environment. 20(4): 126-132.
Lorini, I and Galley, D.J. 2000. Effect of the Synergists Piperonyl butoxide and DEF in deltamethrin resistance on strains of Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). Anais da Sociedade Entomológica do Brasil. 29(4): 749-755.
Manjunath, G.N and Mallapur, C.P. 2015. Studies on population dynamics of spotted pod borer, Maruca testulalis Gey. In blackgram. Karnataka Journal of Agricultural Sciences. 28(3): 418-419.
Nene, Y.L. 2006. Indian pulses through the millennia. Asian Agri- History. 10: 179-202.
Ramanujan, B. 2004. Biological options for insect pests and nematode management. Kalyani Publishers, New Delhi, India. 487
Sohail, A and Irfanullah, M. 2004. Toxicity of dichlorvos 50EC and chlorpyrifos 40EC against house fly, Musca domestica L. Pakistan Journal of Agricultural Sciences. 41(1&2): 65-71.
Sreelakshmi, P., Paul, A., Antu, M., Sheela, M.S and George, T. 2015. Assessment of insecticide resistance in field populations of spotted pod borer, Maruca vitrata fabricius on vegetable cowpea. International Journal of Farm Sciences. 5(3): 159-164.
Wang, D., Qiu, X., Ren, X., Zhang, W and Wang, K. 2009. Effects of spinosad on Helicoverpa armigera (Lepidoptera: Noctuidae) from China: tolerance status, synergism and enzymatic responses. Pest Management Science. 65: 1040-1046.
Wang, X., Huanga, Q., Haoa, Q., Rana, S., Wub, Y., Cuic, P., Yangd, J., Jianga, C and Yanga, Q. 2018. Insecticide resistance and enhanced cytochrome P450 monooxygenase activity in field populations of Spodoptera litura from Sichuan, China. Crop Protection. 106: 110–116.
Yu, S.J. 1983. Age Variation in insecticide susceptibility and detoxification capacity of fall armyworm (Lepidoptera: Noctuidae) larvae. Journal of Economic Entomology. 76: 219-222.
- Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
- Brand Preference of Farmers for Maize Seed
- Issues That Consumer Experience Towards Online Food Delivery (Ofd) Services in Tirupati City
- Influence of High Density Planting on Yield Parameters of Super Early and Mid Early Varieties of Redgram (Cajanus Cajan (L.) Millsp.)
- Influence of Iron, Zinc and Supplemental N P K on Yield and Yield Attributes of Dry Direct Sown Rice
- Effect of Soil and Foliar Application of Nutrients on the Performance of Bold Seeded Groundnut (Arachis Hypogaea L.)

