Micropropagation of Four Sugarcane Varieties Using Shoot Tip, Leaf Roll and Apical Meristem Explants.
0 Views
S. RAJUNAIKA, M. HEMANTHKUMARB, N.V. NAIDUC, P. LATHAD
aPost Graduate student, Department of Genetics and Plant Breeding, S.V. Agricultural College, ANGRAU,
Tirupati – 517502. Chittoor (District), Andhra Pradesh
ABSTRACT
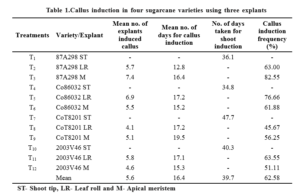
Four sugarcane varieties (87A298, Co86032, CoT8201 and 2003V46) and three explants (shoot tip, leaf roll and apicalmeristem) were used for micropropagation and observations were made on callus, shoot and root induction. Among all fourvarieties 87A298 responded well for callus, shoot and root induction and a opposite performance was observed for CoT8201.
KEYWORDS:
Sugarcane, micropropagation
INTRODUCTION
Sugarcane (Saccharum officinarum L.) is an important agricultural cash crop in tropical and sub tropical region of the world and is the major source of sugar, ethanol, biogas, manure, production of electricity and paper..It is the only member of the family Gramineae belong to genus Saccharum in which in vitro propagation are standardized and commercially viable. In sugarcane, production of sufficient quantity of seed material of a new variety takes several years (8-10 years) if multiplied through conventional methods of seed multiplication, by the time the varieties start deterioration in yield potential. There are also chances of perpetuation of sett-borne diseases. Invitro micropropagation technique is becoming useful in rapid
multiplication of virus free materials through apical meristem culture (Hendre et al., 1983) and callus culture (Ali et al., 2007). Several investigators have suggested various protocols during the past three decades for in vitro micropropagation of sugarcane varieties (Jadhav et al.,2001; Pawar et al., 2002; Sengar et al., 2012; Chaudhary et al., 2013).
The present study was therefore undertaken to study invitro micropropagation of four sugarcane vari-eties (87A298, Co86032, CoT8201 and 2003V46) using
three explants (shoot tip, leaf roll and apical meristem).
MATERIALS AND METHODS
Explant source
Healthy tops of 87A298, Co86032, CoT8201 and 2003V46 were collected from the field grown plants of sugarcane (Saccharum officinarum L.) maintained at Agricultural Research Station, Perumallapelle, Tirupati.
Surface sterilization of explants Actively growing points of sugarcane top, taken as explants from 8-12 months old sugarcane cultivar.Outer whorls of mature leaves were removed till yellowish white covering around apical meristems appear. After removing outer whorls of leaves, the tops are sized to 10 cm length by cutting off at the two ends. These were then washed with sterile distilled water thrice and then treated with 3 per cent (w/v) sodium hypochlorite (HgCl2) solution for about 10 minutes in laminar air flow chamber and washed out thrice with sterile distilled water. The explants (apical shoots) are picked carefully with sterile foreceps and placed in a sterile petri dish. Using a fine forceps and scalpel, the outer leaf sheaths are removed one by one without exerting pressure on the internal tissues. The process is repeated until the apical dome with two or three leaf primordia is exposed.
ter excising the apex, the explants are transferred immediately on a filter paper support immersed in MS medium supplemented with different concentrations of growth regulators. All the above operations were performed under aseptic conditions in laminar airflow cabinet.
Culture medium and condition
All the three explants of four varieties were inoculated on to sterilized semisolid basal MS medium (Murashige & Skoog’s, 1962) supplemented with different concentrations and combinations of different plant growth regulators.
Callus induction
MS medium supplemented with 2, 4-D (3.0 mg l-1) was used for callus induction.
Shoot regeneration medium
White friable calli were cultured on MS medium supplemented with BAP (3 mg l-1), IAA (2 mg l-1) and Kinetin (2 mg l-1) were used for shoot regeneration. It was sub-cultured every 7 days of incubation.
Rooting medium
For root induction, elongated micro shoots measuring about 5-6 cm in length were excised from culture tube and transferred to half strength MS medium supplemented with NAA (2 mg l-1) and sucrose (30 g l-1).
Environmental condition
In medium, the pH was adjusted in the direction of 5.8 and autoclaved for 15-20 minutes at 121oC and at 15 lbs psi. The cultures were incubated with 16 hour of light and 8 hour of dark (fluorescent tubes are used as a light source), with artificial illumination of 2000-3000 lux by placing the cultures at 25-30cm below the fluorescent light and maintained the temperature at 25 ± 2oC. Sub cultures were done for every 2-3 weeks according to the need of the experiment.
Acclimatization and transfer of plantlets to soil
Plantlets with well developed roots were removed from the culture medium. Washed the roots gently under running tap water and were transferred to poly bags for
hardening which contain autoclaved farm yard manure, soil and sand (1:1:1). The harden plantlets were covered with porous polythene sheets for maintaining high humidity and were kept under shade in a net house for further growth and development. After 30 days the plantlets were transplanted in to the soil in field condition.
Statistical analysis
Completely Randomized Design (CBD) was set up in an experiment with three replications. In each replication, 10 explants were used per treatment. Observations were recorded in terms of number of explants induced callus, number of days taken for callus induction, callus induction percentage, time taken for shoot induction, number of days for shoot initiation, shoot regeneration frequency, number of shoots per explant, average shoot length (cm), time taken for root induction, root induction frequency, number of roots produced per shoot, average root length (cm), number of days for acclimatization and survival percentage.
RESULTS AND DISCUSSION
Callus induction frequency in the leaf rolls of Co86032 and 2003V46 was higher (76.66% and 63.55%) and took more number of days (6.9 and 5.8) for callus induction than apical meristem cultures (Table 1.) However reverse was true with respect to 87A298 and CoT8201. These results were consistent with the report of Kaur and Kapoor (2016), Dinesh et al., (2015), Abu et al. (2014) and Tiwari et al., (2013) who studied in vitro micropropagation in various sugarcane varieties and found that 3 mg l-1 of 2, 4-D was the best concentration for callus induction. Maximum of 70.7 ± 8.1 per cent explants showed callus initiation on MS medium containing 3.0 mg l-1 of 2, 4-D (Goel et al., 2010). Dash et al., (2011) reported the highest callus induction frequency of 95 per cent from shoot tip at 3 mg l-1 of 2, 4-D.
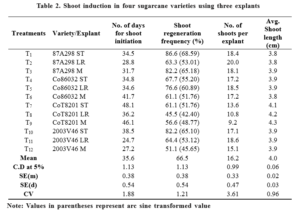
In general shoot regeneration frequency was higher in shoot tip explants of the varieties with an exception to Co86032, where in leaf roll explant exhibited higher shoot regeneration frequency (76.6%) than the other explants (Table 2.). The time taken for shoot initiation was more in shoot tip derived explants than the other explants of respective varieties except Co86032 apical meristem (41.7 days). Leaf roll explants of all the varieties took less time for shoot initiation. Similarly leaf roll explants
produced more number of shoots per explant except CoT8201 shoot tip (13.6). Garcia et al., (2007) observed shoot regeneration after 30 days of inoculation. Biradar et al. (2009) and Godheja et al., (2014) reported the shoot induction within 7-10 days of culture.
Yadav et al., (2013) recorded maximum of 53.3
± 2.7 per cent and 51.1 ± 3.2 per cent shoot initiation was recorded in CoSe01235 and CoS99259, respectively for shoot tip explant and 35.2 ± 3.4 per cent and 34.3 ± 3.9 per cent for meristem culture on MS medium containing BAP and Kinetin (0.5 mg l-1 each). Tripathi and Lal (2013) observed shoot regeneration of 80 per cent, 65 per cent and 40 per cent in shoot tip, leaf roll and bud explants respectively. Sughra et al., (2014) observed average number of 6.50, 4.70 and 3.80 shoots per explant in BL-4, Thatta-10 and Larkana-2001 respectively. Yadav et al., (2013) obtained 5.8 cm and 5.6 cm shoot length in CoSe 01235 and CoS 99259 respectively.
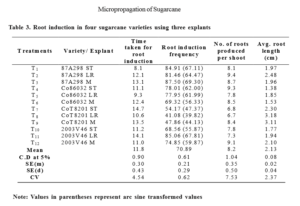
Apical meristem of 87A298 and Co86032 took more time (13.1 days and 12.4 days) for root induction with high root induction frequency (87.50% and 69.32%) (Table3). Where as in shoot tip explant of CoT8201 and leaf roll explant of 2003V46 recorded higher root induction frequency (54.17% and 85.06%) than the other explants of the respective varieties and took more time for root induction (9.3 days and 14.1 days). These results were supported by Sood et al., (2000) reported root induction in 10 -15 days on MS medium + 7 mg l-1 NAA. On contrary, Sughra et al. (2014) observed root induction of
9 days, 10 days and 11.50 days in BL-4, Thatta-10 and Larkana-2001 respectively with NAA (3 mg l-1). Abu et al., (2014) recorded 88 per cent rooting response in NCO-334 variety on media supplemented with 4 mg l-1 of NAA and 5 mg l-1 NAA (85.33 per cent) whereas maximum percentage of shoots regenerated roots at 1 mg l-1 IBA and 1.5 mg l-1 IBA (85 per cent) for B52-298 variety. According to Tolera (2016) sugarcane variety B41-227 gave maximum (33 ± 0.15) number of roots per shoot, while only 24.17 ± 0.00 roots per shoot were observed in N14 on half strength MS medium with 2 mg l-1 NAA and 1 mg l-l IBA. Sughra et al., (2014) recorded average number of 6.80, 2.5 and 4.90 roots per shoot in BL-4, Thatta-10 and Larkana-2001 respectively with 3 mg-1 NAA. Tolera (2016) obtained average root length of 2.92
± 0.18 cm and 2.58 ± 0.00 cm in B41-227 and N14 respectively. Behera and Sahoo (2009) obtained average root length of 4.0 ± 0.94 cm for the variety Nayana on
MS media supplemented with 2.5 mg l-1 of NAA. Gopitha et al., (2010) recorded average length of root 4.9 cm in half MS medium supplemented with 3 mg l-1 of NAA.
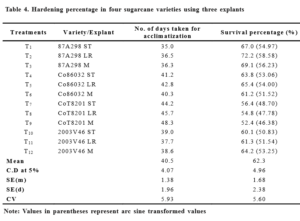
Seedlings obtained from leaf roll explants of 87A298 and Co86032 survived better (72.2% and 65.4%) when compared with their counterparts (Table 4). Where as in CoT8201 shoot tip derived explants and in 2003V46 apical meristem derived explants recorded higher survival per cent (56.4% and 64.2%) than the other explants. Whereas, Purushothaman et al., (2000) reported 42 days for acclimatization with high survival percentage on sand, silt and press mud in a ratio of 1:1:1.
CONCLUSIONS
Differential response for callus induction, shoot induction and root induction was observed among four varieties with three explants. Among all four varieties, 87A298 responded well and CoT8201 did not give better results for callus induction, shoot induction, root induction and hardening. CoT8201 require a standard protocol for in better in vitro response. Hence, by using micropropagation best performing genotypes can be multiplied and commercialized within a short period of time and supplement the conventional propagation which improves both the quality and quantity of the planting materials.
REFERENCES
- Abu, G., Mekbib, F and Teklewold, A. 2014. Effect of genotype on in vitro propagation of elite sugarcane (Saccharum offinarum L.) varieties of Ethiopian sugar estates. International Journal of Technology Enhancements and Emerging Engineering Research. 2(6): 123-128.
- Ali, A., Shagufta, N and Iqbal, J. 2007. Effect of different explants and media composition for efficient somatic embryogenesis in sugarcane (Sacchraum officinarum L.).Pakistan Journal of Botany. 39(6): 1961-1971.
- Behera, K. K and Sahoo, S. 2009. Rapid in vitro micro Propagation of sugarcane (Saccharum officinarum L. cv- nayana) through callus culture. Nature and Science 7(4):1-10.
- Biradar, S., Biradar, D.P., Patil, V.C., Patil, S.S and Kambar, N.S. 2009. Invitro plant regeneration using shoot tip culture in commercial cultivar of sugarcane. Karnataka Journal of Agriculture Science. 22(1):21-24.
- Chaudhary, R., Sanjeev, K and Sengar, R.S. 2013. Effect of Auxins on rooting of Invitro raised shoots of two different sugarcane varieties. Vegetos. 26(2):444-446.
- Dash, M., Mishra, P and Mohapatra, D. 2011. Mass propagation via shoot tip culture and detection of genetic variability of Saccharum officinarum clones using biochemical markers. Asian Journal of Biotechnology. 3(5): 378-387.
- Dinesh, P., Thirunavukkarasu, P., Saraniya, A. R and Ramanathan, T. 2015. Invitro studies of sugarcane variety Co-91017 through micropropagation of
- shoot tip culture Advances in Plants & Agricultural
- Garcia, R., Cidade, D., Castellar, A., Lips, A., Magioli, C., Callado, C and Mansur, E. 2007.In vitro morphogenesis patterns from shoot apices of sugarcane are determined by light and type of growth regulator. Plant cell Tissue and Organ Culture. 90(2): 181-190.
- Godheja, J., Sudhir, K., Sekhar and Modi, D. R. 2014. The standardization of protocol for large scale production of sugarcane (Co86032) through micropropagation. International Journal of Plant, Animal and Environmental Sciences. 4(4): 135-143.
- Goel, Y., Singh, V.P., Lal, M and Sharma M.L. 2010.In vitro morphogenesis in leaf sheath explants of sugarcane hybrid var. CoS 99259. Sugar Tech. 12(2): 172-175.
- Gopitha, K., Bhavani, A.L and Senthilmanickam, J. 2010. Effect of the different auxins and cytokinins in callus induction, shoot, root regeneration in sugarcane. International Journal of Pharma and Bio Sciences. 1(3): 1-7.
- Hendre, R.R., Iyer, R.S., Kotwal, M., Khuspe, S.S and Mascarenhas, A.F. 1983. Rapid multiplication of sugarcane by tissue culture. Sugarcane 1:5-8.
- Jadhav, A.B., Vaida, E.R., Aher, V.B. and Pawar, A.M. 2001. In vitro multiplication of Co86032 sugarcane (Saccharum officinarum) hybrid. India Journal of Agricultural Sciences. 71(2): 113-115.
- Kaur, R and Kapoor, M. 2016. Plant regeneration through somatic embryogenesis in Sugarcane. Sugar Tech. 18(1): 93-99.
- Murashige and Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiological Plantarum.15, 473-497.
- Pawar, S.V., Patil, S.C., Jambhale, V.M and Mehetre, S.S. 2002.Effect of growth regulators on invitro multiplication of sugarcane varieties. Indian Sugar.109-111.
- Purushothaman, R.S., Babu, C and Giridharan, S. 2000. Micropropagation (Tissue culture) of sugarcane varieties for seed production. South Indian Sugar cane and Sugar Technologists Association (SISSTA). 25:35-37
- Sengar, R.S., Sengar, K and Kumar, G.S.2012. Biotechnological intervention for boostingsugarcane production. Research Journal Agricultural Sciences. 3(3):593-599.
- Sood, N., Kanwar, R.S., Goswal, S.S and Singh, S.2000. Efficient protocol for micropropagation of sugarcane variety COJ 85. South Indian Sugarcane and Sugar Technologists Association (SISSTA).25:3537.
- Sughra, M.G., Altaf, S.A., Rafique, R.M and Muhammad, M.S. 2014. In vitro regenerability of different sugarcane (Saccharum officinarum L.) varieties through shoot tip culture. Pakistan Journal of Biotechnology. 11(1): 13-23.
- Tiwari, S.,Arya, A., Yadav, P., Kumari, Pand Kumar, S. 2013. Enhanced in vitro regeneration of two sugarcane varieties COS 8820 and COS 767 through organogenesis. Indian Journal of Fundamental and Applied Life Sciences. 3(4):17-26.
- Tolera, B. 2016.Effects of Naphthalene Acetic Acid (NAA) and Indole -3- Butyric Acid (IBA) on in vitro rooting of sugarcane (Saccharum officinarum L.) micro shoots. Journal of Biotechnology & Biomaterials.6:1.
- Tripathi, S and Lal, M. 2013. Developing micropropagation protocol for mass multiplication of sugarcane variety CoS 07250. Agrica. 1(2): 67-70.
- Yadav, S and Ahmad A. 2013.Standardisation of callus culture techniques for efficient sugarcane micropropagation. Cibtech Journal of BioProtocols. 2(2): 29-32.
- Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
- Brand Preference of Farmers for Maize Seed
- Issues That Consumer Experience Towards Online Food Delivery (Ofd) Services in Tirupati City
- Influence of High Density Planting on Yield Parameters of Super Early and Mid Early Varieties of Redgram (Cajanus Cajan (L.) Millsp.)
- Influence of Iron, Zinc and Supplemental N P K on Yield and Yield Attributes of Dry Direct Sown Rice
- Effect of Soil and Foliar Application of Nutrients on the Performance of Bold Seeded Groundnut (Arachis Hypogaea L.)

