IN VITRO SCREENING OF BIO EFFICACY OF DIFFERENT ISOLATES OF Trichoderma viride AND Pseudomonas fluorescens ON THE MYCELIAL GROWTH OF Sclerotium rolfsii INCITANT OF GROUND NUT STEM ROT.
0 Views
B. RAGHAVENDRA, T. SRINIVAS AND B. PADMODAYA
Department of Plant Pathology, S. V. Agricultural college, ANGRAU, Tirupati-517 502, Chittoor Dt., Andhra Pradesh, India.
ABSTRACT
The antagonistic effect of three isolates Tr-DM, Tr-SNG and Tr-RGP of Trichoderma viride and three isolates Pf-DM, Pf-SNG and Pf-RGP of Pseudomonas fluorescens against Sclerotium rolfsii were assessed by dual culture technique in vitro. The isolates of fungal agent T. viride were more effective in controlling the mycelial growth of S. rolfsii when compared with isolates of bacterial bio control agent P. fluorescens. Among the fungal bio-control agents maximum inhibition (87.18%) of mycelial growth of S. rolfsii was observed with the DM-Tr isolate of T. viride and among bacterial isolates maximum inhibition (28.25%) of mycelial
growth of S. rolfsii was observed with the Pf-DM isolate of P. fluorescens.
KEYWORDS:
Bio agents, dual culture, inhibition, Trichoderma viride and Pseudomonas fluorescens, Sclerotium rolfsii.
INTRODUCTION:
Groundnut (Arachis hypogaea L.) is an important oil seed crop suitable for cultivation in tropical areas of the world. It is regarded as “King of oilseed crops” on account of its diversified uses. Groundnut is third largest oil seed crop grown in world and second in India. Ground-nut seeds are rich in oil (43-55%) as well as protein (25-28%) and also contains 18 percent carbohydrates. It can supply about 5.6 and 5.8 calories pergram of kernel in the raw and roasted forms respectively. It is also very good source of minerals (calcium, magnesium and iron) and vi-tamins (B1,B2 and Niacin). Groundnut being a legume crop, it fixes a large amount of nitrogen and improves the fertility status of the soil. Groundnut cake is used as ani-mal feed and the shell sometimes used as fodder.
S. rolfsii was first reported on tomato by Rolfs (1892) later the pathogen was named as Sclerotium rolfsii by Saccardo (1911). Higgins (1927) worked in detail on physiology and parasitism of S. rolfsii. This was the first detailed and comprehensive study in USA. It is distrib-uted in tropical and subtropical regions of the world where bean, lima bean, onion, garden bean, pepper, potato, tem-peratures prevail. The fungus has a wide host rang po-tato, omato and water melon (Aycock, 1966). 500 speciesin about 100 families including ground.
Accoding to West (1961) the fungus belonging to the group of non-spore producing fungi and production of small round sclerotia is an important morphological char-acteristic feature of the organism. Initially the fungal myce-lium is silky white in pure culture but gradually looses its luster and becomes somewhat dull in appearance. The mycelium is radiating with abundant aerial hyphae. The mycelum completely disappears over a period of three months leaving only sclerotial bodies. According to (Subramanian 1964 and Mehan et al., 1995) sclerotial are at first white, becomes light brown to dark brown at ma-turity.
Stem rot of groundnut has become one of the major constraints in recent years. Management of stem rot dis-ease is difficult because of soil borne nature and the chemi-cal methods are very expensive and will not have good effect against the pathogen. In view of unsatisfactory management of this disease, a considerable attention has been given on the other non-chemical means of plant dis-ease control i.e., the integration of biological methods which include the use of eco-friendly bio control agents. Virupaksha et al., (1997) reported the antagonistic activ-ity of Trichoderma harzianum and Trichoderma virideagainst Sclerotium rolfsii and found to be effec-tive in inhibiting the mycelial growth and reducing pro-duction of sclerotial bodies irrespective of inoculation pe-
riods.
against Sclerotium rolfsii and found to be effective in inhibiting the mycelial growth and reducing production of sclerotial bodies irrespective of inoculation periods.
MATERIALAND METHODS:
List of different isolates of bio agents used in the study
Three isolates of T. Viride available at Plant Pathology lab, S.V.Agricultural College, Tirupati were evaluated in in vitro against S. rolfsii by dual culture method (Dennis and Webster, 1971). Twenty ml of sterilized PDA was poured in to Petri plate of 9 cm diameter aseptically, Mycelial discs measuring 6 mm diameter from four day old cultures of both fungal antagonist and the test pathogen were inoculated 7 cm apart (leaving 1 cm from periphery). Four replications were maintained for each treatment. Radial growth of the mycelium was recorded and percent inhibition over control was calculated. The data were analysed statistically using Completely Randomized Design (CRD)
- C T X100 C
Where,
I = Per cent reduction in growth of test pathogen C= Radial growth (mm) in monocultured check T= Radial growth (mm) in dual cultured plates
Evaluation of efficacy of P. fluorescens isolates Evaluation of efficacy of P. fluorescens available at Plant Pathology lab, S.V.Agricultural College, Tirupati were evaluated in vitro against S. rolfsii by dual culture method (Dennis and Webster, 1971). S. rolfsii was nocultated at
ithe center of PDA plate. Test bacterial cultures were str -eaked individually on both the sides of the S. rolfsii at 2.5
cm distance leaving 2.0 cm periphery. Plates inoculated with S. rolfsii alone were maintained as checks. Inocu-lated plates were incubated at 28 2oC. Four replica-tions were maintained for each treatment. Observations were recorded considering zone of inhibition up to four days when S. rolfsii completely occupied the plate in monoculture check. Per cent inhibition of mycelia growth over control was calculated using the formula given. In the data was analyzed statistically using CRD design.
RESULTSAND DISCUSSION
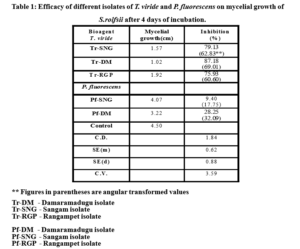
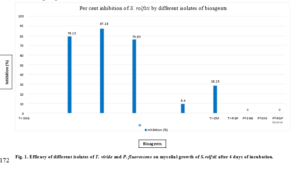
Effect of different isolates of Trichoderma viride and Pseudomonas fluorescens were presented in Table 1 and Fig 1.
Significant differences were observed among the three isolates of T. viride in inhibiting the mycelial growth of S. rolfsii. Maximum percent inhibition (87.18%) myce-lial growth was observed in Tr-DM isolate followed by Tr-SNG isolate (79.13%). Tr-RGP isolate proved to be
least effective (75.93%) among the three isolates.
Among the three T. viride fungal bio agents and three bacterial P. fluorescens bio agents, fungal bio agents proved to be significantly highly effective on mycelia growth of S. rolfsii compared to bacterial bio agents.
Antagonistic activity of T. harzianum and T. viride against S. rolfsii was found to be effective in inhibiting the mycelial growth and reducing production of sclerotial bodies was reported by Virupaksha et al., (1997) and Rajalakshmi (2002), Salvi et al., (2017) screened three bio-agents in vitro namely T. viride, T. harzianum and P. fluorescens and found that T. viride showed maximum growth inhibition (83.33%) against S. rolfsii. Ganesan and Gnanamanikyam (1987) and Wokocha et al., (1986) reported that, the native strain of P. fluorescens restricted the growth of S. rolfsii causing stem rot of groundnut. Chanutsa et al., (2014) reported 100 per cent inhibition in growth of S. rolfsii with culture filtrate of P. fluorescens
SUMMARYAND CONCLUSION:
The antagonistic effect of three isolates Tr-DM, Tr-SNG and Tr-RGP of Trichoderma viride and Pf-DM, Pf-SNG and Pf-RGP of Pseudomonas fluorescens against S. rolfsii were assessed by dual culture technique in vitro. The isolates of fungal biocontrol agent T. viride were more effective in controlling the mycelial growth of S. rolfsii when compared with isolates of bacterial biocontrol agent P. fluorescens. Among the fungal biocontrol agents maximum inhibition (87.18%) of myce-lial growth of S. rolfsii was observed with the Tr-DM isolate of T. viride and among bacterial isolates maximum inhibition (28.25%) of mycelial growth of S. rolfsii was observed with the Pf-DM isolate of P. fluorescens and they were significantly different from each other.
LITERATURE CITED:
- Aycock, R. 1966. Stem rots and other disease caused by Sclerotium rolfssi. North Carolina Agri – cultural Experiment Station Technical Bulle tin No. 174, p.202.
- Chanutsa, N., Phonkerd, N and Bunyatratchata, W. 2014. Potential of Pseudomonas aeruginosa to con-trol Sclerotium rolfsii Causing Stem Rot and Collar rot Disease of Tomato. Journal of Ad-vanced Agricultural Technologies 1(2):132-135.
- Dennis, C and Webster, J. 1971. Antagonistic property of species group of Trichoderma production of non volatile antibiotics. Transaction of British My-cological Society. 57(1); 25-39.
- Ganesan, P and Gnanamanikyam, S.S. 1987. Biological control of Sclerotium rolfsii (Sacc.) in peanut by inoculation with Pseudomonas fluroscens. Soil Biology and Biochemistry. 19:35-38.
- Higgins, B.B. 1927 Physiological and parasitism of Scle-rotium rolfsii (Sacc.). Phytopathology. 17:417-448.
- Salvi, P. P., Pande, V. S., Pawar, S. V and Joshi, P. V. 2017. Effect of different fungicides and bio con-trol agents against Sclerotium rolfsii Sacc. Caus-ing collar rot and root rot of pigeon pea under in vitro condition. International Journal of Chemi-cal Studies 5(6): 1494-1496.
- Subramanian, K. S. 1964. Studies on sclerotial root rot disease of groundnut (Arachis hypogea L.) by Sclerotium rolfssi sacc. Madras Agricultural Journal. 51: 367-368.
- Virupaksha, P. H., Hiremath, P. C and Patil, M. S., 1997. Biological control of collar rot of cotton caused by Sclerotium rolfsii sacc Karnataka Journal of Agricultural Sciences, 10: 397-403.
- West, 1961. Sclerotium rolfsii History, Taxonomy, Host range and distribution. Phytopathology. 51: 108-109.
- Wokocha, R. C., Ebenere, A. C and Erinle, I.D. 1986. Biological control of the basal stem rot disease of tomato caused by Corticium rolfssi (Sacc.) Curzi in Northern Nigeria. Tropical Pest Management, 32: 35-39.
- Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
- Brand Preference of Farmers for Maize Seed
- Issues That Consumer Experience Towards Online Food Delivery (Ofd) Services in Tirupati City
- Influence of High Density Planting on Yield Parameters of Super Early and Mid Early Varieties of Redgram (Cajanus Cajan (L.) Millsp.)
- Influence of Iron, Zinc and Supplemental N P K on Yield and Yield Attributes of Dry Direct Sown Rice
- Effect of Soil and Foliar Application of Nutrients on the Performance of Bold Seeded Groundnut (Arachis Hypogaea L.)

