Technology Advances in Seed Priming (Seed Priming: A Tool for Quality Seed Production)
0 Views
A. ANUSHA REDDY*, B. RUPESH KUMAR REDDY, S. VASUNDHARA AND R. NARASIMHULU
Department of Seed Science and Technology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
The critical stages during early crop growth are uniform seed germination, early seedling growth, and uniform plant stand. Low crop yield is attributed to uneven seed germination and seedling growth. Therefore, the quality of seed can be improved through priming in addition to the field management techniques for better seed germination. Priming is a physiological technique of controlled seed hydration and drying to enhance the pre-germinative metabolic process for rapid germination, seedling growth and yield under normal as well as stressed conditions. The primed seeds show faster and uniform seed germination due to different enzyme activation, metabolic activities, biochemical process of cell repair, protein synthesis and improvement of the antioxidant defence system as compared to unprimed seeds. There are many techniques of seed priming which are broadly divided into conventional methods and advanced methods. However, priming is strongly affected by various factors such as temperature, aeration, light, priming duration and seed characteristics. The present article highlights the priming mechanism and the available technologies as a tool for superficial seed germination and crop stand..
KEYWORDS: .
INTRODUCTION
Seed plays a crucial role in agriculture since ancient times. It is the starting point, first determinant of the future plant development and the most important factor for successful production. Various techniques are available, which have the potential to improve emergence and stand establishment under wide range of field environments. Seed priming is one of the physiological ways which enhances performance of seed and show rapid and synchronized germination.
SEED PRIMING
It is a pre-sowing treatment in which seeds are soaked in osmotic solution that allows the seeds to imbibe water and go through the first stages of germination but does not permit radicle protrusion through the seed coat. It is based on the principle of controlled imbibition, to a level that permits pre-germination metabolism to proceed but prevents actual emergence of radicle (McDonald. 2000).
PHENOMENA OF SEED PRIMING
After sowing, usually the seeds remain in soil for some time for absorbing water and nutrients required for their growth. In seed priming technique this time is reduced and the seed germinate quickly and uniformly. Added to the hydration, the priming also decreases the seed sensitivity to the external environmental factors. (Afzal et al., 2016). Priming promotes seed germination under three stages such as imbibition, germination, and growth. During the imbibition stage, the water uptake promotes protein synthesis and respiratory activities through messenger ribonucleic acid (mRNA). The second stage is related to the initiation of different physiological activities related to germination such as protein synthesis, mitochondria synthesis and alteration in soluble sugars (Varier et al., 2010). The critical factor during seed priming is the controlled water uptake during the second stage, before the emergence and growth of radical from the seed coat during the last stage. The second stage (germination) is much sensitive to environmental factors than the third stage (Côme and Thévenot, 1982). Therefore, during priming, the seeds that have passed through the second stage could germinate under variant environmental conditions as compared to unprimed seeds (Corbineau and Côme, 2006).
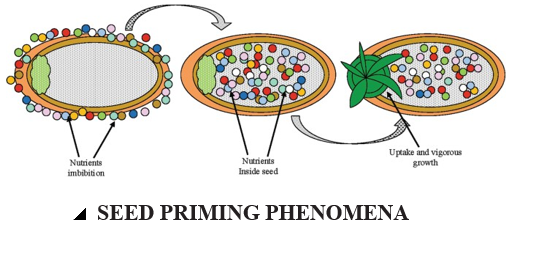
SEED PRIMING PROCESS
Priming allows some of the metabolic processes necessary for germination to occur without actual germination of the seed takes place. This prevents the seeds from absorbing enough water for radicle protrusion, thus suspending the seeds in the lag phase. This hydration is sufficient to permit pre-germinative metabolic events but insufficient to allow radicle protrusion through the seed coat (Taylor et al., 1998).
SEED PRIMING
PHYSIOLOGICAL & BIOCHEMICAL BASIS
When seeds are planted in cold soils, imbibitional damage is reduced. Conversely, when seeds are planted in extremely warm soils, secondary dormancy is reduced. This is accomplished through seed priming. The primed seeds leak less metabolites, seed endosperm is hydrolyzed during priming that permits faster embryo growth and cell wall elasticity is increased. Hydration of the seed during priming permits early DNA replication, increased RNA & protein synthesis and more ATP availability. Increase in seed vigour may also occur following seed priming, as repair of deteriorated parts of seed occurs during the hydration phase of the process (Basra et al., 2005, Farooq et al., 2006, Khan et al., 1978, Zhang et al., 2007).
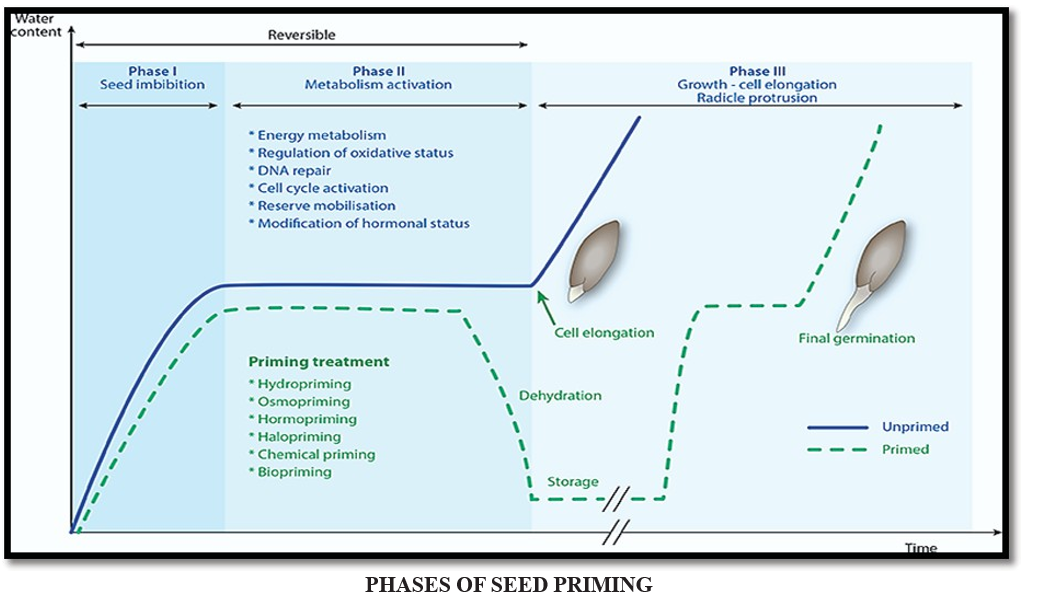
Stages of water uptake during germination where priming is relevant
The pattern of water uptake during priming is similar to that during germination but the rate of uptake is slower and controlled to prevent radicle emergence.
Changes in protein profile
Proteomic analysis in Arabidopsis revealed that new proteins are involved either in the imbibition process of the seeds (such as an actin isoform or a WD-40 repeat protein) or in the seed dehydration process (e.g., cytosolic glyceraldehyde-3-phosphate dehydrogenase) which helps to characterize seed vigor of commercial seed lots and to develop and monitor priming treatments.
Enzyme activation in relation to priming
Increases in activities of alpha-amylase in rice, acid phosphatase and esterase in lettuce, and antioxidant enzymes in Lucerne. A rapid resumption of DNA synthesis and initiation of cell division in wheat soon after hydration. Repair of DNA and other cellular components (e.g., membranes), which may be damaged during seed maturation, dehydration.
METHODS OF SEED PRIMING
There are several techniques of seed priming that are broadly divided into conventional and advanced methods. The traditional techniques are comprised of hydropriming, osmo-priming, halo-priming, drum- priming, bio-priming, solid matrix priming, whereas the advanced techniques of seed priming include nano- priming, hormonal-priming, magneto-priming. These techniques are described below.
OSMO PRIMING
Osmotic priming of seeds also known as Osmopriming or osmoconditioning describes incubation of seeds with aerated solutions of low water potential which are rinsed off afterwards. e.g., polyethylene glycol (PEG), glycerol, sorbitol, or mannitol are used as osmoticants to create low water potential solutions. Osmopriming essentially exposes seeds to a low water potential to restrict the rate and extent of imbibition. (Heydecker et al., 1973).
Effects of Osmo – priming
Wild rye (Leymus chinensis L.) seed priming with 30% PEG for 24 h resulted in increase in the cell activity of superoxide dismutase, peroxidase and a rapid increase in the respiratory intensity which were associated with an increase in germination and vigour (Jie et al., 2002).
Chickpea seed primed with water, 2 and 4 per cent mannitol increased the length and biomass of roots and shoots of seedlings as compared to non-primed control under salt stress conditions. (Kaur et al., 2002 and 2005)
HALOPRIMING
Halopriming refers to soaking of seeds in solution of inorganic salts i.e., NaCl, KNO3, KCl, KH2PO4, CaCl2, CaSO4 etc. (Batool et al., 2014).
Effect of Halo-priming:
Chickpea seeds primed in 0.05% solution of ZnSO4.7H2O (zinc sulphate heptahydrate) gave a 19 per cent high seeds production and have 29 per cent more seeds Zn as compared to that of nonprimed chickpea seeds (Harris et al., 2008).
Seed primed with sodium molybdate dihydrate (0.02% and 0.04%) for 5 h improved the yield of mung bean (Umair et al., 2011).
HYDROPRIMING
Hydropriming involves soaking of seeds in water before sowing and may or may not be followed by air drying of the seeds. Seeds are immersed in sterilized distilled water maintained at appropriate temperature and the duration of hydro-priming is determined by controlling seed imbibition during germination.
Effects of Hydro – priming
- Enhancement of physiological and biochemical events taking place in rice seeds even when the germination is suspended by low osmotic potential and negligible matric potential of the imbibing medium (Basra et al., 2005).
- A simple, economical and a safe technique for increasing the capacity of seeds towards osmotic adjustment, enhancing seedling establishment and crop production under stressed conditions (Kaur et al., 2002).
DRUM PRIMING
This is misting of seed with water and re-drying before they complete germination. Seeds are rotated in a drum with specific amount of water introduced as a fine mist. As the seeds absorb water, a sensitive scale monitors increase in seed weight until the desired wet weight is achieved.
BIO-PRIMING
Bio-priming is a process of biological seed treatment that refers to combination of seed hydration (physiological aspect of disease control) and inoculation (biological aspect of disease control) of seed with beneficial organism to protect seed. It is an ecological approach using either bacteria or selected fungal antagonists against the soil and seed-borne pathogens.
Effects of Bio – priming
- The mutual response of both Trichoderma harzianum and Pseudomonas fluorescens, when applied on pepper seeds as bio-priming agent, resulted in a significant growth of seedlings (Kumar et al., 2010; Reddy, 2012).
- Within the roots of tomato and rice plants, mycorrhizal fungi activate the aggregation of several transcripts and proteins which predicts the function of the plant defence mechanism (Pozo and Azcon- Aguilar, 2007).
SOLID MATRIX PRIMING
It is a process in which moistened seeds are mixed with an organic carrier and the moisture content of the mixture is brought to a level just below the level of the seed germination.
HORMONAL PRIMING
Hormonal priming is the pre-seed treatment with different hormones i.e., salicylic acid, ascorbate, kinetin, etc. which promote the growth and development of the seedlings. The seeds are soaked in aerated solution of hormones like GA, kinetin, ABA, proline and salicylic acid.
Effects of Hormonal priming
- Sorghum seed primed with gibberellic acid, salicylic acid and ascorbic acid increased germination characteristics of aged seed. Antioxidant activity of aged seeds increased after seed priming. (Azadi et al., 2013)
NANO PRIMING
It is the process of seed priming in which seeds are primed with the nano particles like Sio2, Tio2 and silver. Nano-particles enhance water uptake in seeds and activate enzymatic and hormonal responses during seed germination and plant growth.
Effects of Nano-priming
- AgNPs primed wheat and barley seeds show significant increase in the germination percentage, results showed that use of nanoparticles increased extent of seedling vigor index in plants compared to the control (Abou-Zeid and Moustafa, 2014).
MAGNETO PRIMING
Magneto seed priming involves exposure of seed to a magnetic field.
Effects of Magneto- priming
- SMF primed seeds were exposed to pulsed magnetic field (PMF) dose in the cycles of 2, 3, 5 or 6 min on and off where PMF dose of 3 min on and off cycle showed substantial enhancement of 23% in seedling vigour compared to other treatments (Gupta et , 2015)
- Magneto-priming of cucumber seed at 200 mT magnetic field for 1 hour improved germination parameters, water uptake, activities of hydrolytic enzymes, amylase and protease and activities of antioxidant enzyme like superoxide dismutase, catalase and glutathione reductase (Bhardwaj et al., 2012).
Factors affecting seed priming
Seed priming is highly affected by various biotic and abiotic factors such as aeration, temperature, time and seed quality. Among these, aeration is the most important and effective factor affecting seed respiration, seed viability and seed emergence/germination (Bujalski and Nienow, 1991; Heydecker et al., 1973). Heydecker and Coolbear (1977) and Bujalski et al. (1989) reported the impact of aeration in seed priming by observing enhanced germination percentage in aerated PEG solution treatment as compared to non-aerated. Similarly, temperature is another important factor influencing the germination of primed seeds. Basra et al. (2005) reported that optimum temperature ranges from 15 to 30°C for most of the seed germination. On the other hand, McDonald (2000) reported slow germination at a lower priming temperature. Wahid et al. (2008) documented a range of 15–20 °C for seed priming, and the duration of priming may extend from almost 8 h to 14 days based on plant species, osmotic solution, osmotic potential and temperature (FinchSavage et al., 1991). Seed quality is another important factor in seed germination and a viable and vigorous seed is the first most necessary for seed priming (Cantliffe, 1987). Other seed characteristics also play a role in seed priming and germination process. For instance, Patanè et al. (2008) reported osmo-priming with PEG solution unsuitable for sorghum seeds priming. Sorghum is rich in tannin that could be removed with the solution during treatment and hence leads to lower seed germination. In this regard, Passam et al. (1989) stated that the salt solution is effective as compared to mannitol and PEG solutions. Similarly, O’Sullivan and Bouw (1984) reported KNO3 and K3PO4 as an effective priming solution in pepper seeds as compared to PEG.
LIMITATIONS AND PERSPECTIVE IN SEED PRIMING TECHNOLOGY
Seed priming has been developed as a promising technology for crop stand in a variety of environmental conditions. However, many protocols such as seed desiccation (redrying) after priming may affect different physiochemical process which reduces seed longevity and viability (Heydecker and Gibbins, 1977; Halmer, 2004). Other conditions for post-treatment such as storage temperature, air composition and moisture also negatively affect seed viability (Schwember and Bradford, 2005). Similarly, the prolonged seed treatment during priming may also cause loss of seed tolerance to desiccation (Sliwinska and Jendrzejczak, 2002). Priming itself in certain circumstances may also cause different problems. For instance, all priming protocols may not lead to significant germination and growth where inappropriate priming conditions may cause degradation of the protective proteins (Capron et al., 2000). Hence, it is critical to select specific priming protocol for different plants about germination and growth in different environmental conditions. Thus, for filling the gap and successful application of priming technology,
detailed studies focusing on treatment technologies, gene expressions and molecular mechanisms need to be fully explored. Correspondingly, the advanced methods of seed priming such as priming with nanoparticles may also have deleterious effects on environment, plant and human health. In this regard, many studies need to be performed for resolving the impact of nanomaterials when enter the food chain by using them in agriculture. Extensive researches are still required for each priming technology in terms of optimal dose, exposure time, and dose rate that could affect plant growth and development.
FUTURE PROSPECTS
Being a cost-effective method, it is a practical and suitable approach to reduce the gap between potential and actual yields, even under stressful conditions. Under stress condition, seed priming is one of the best ways to reduce germination related problems, especially when crops are grown under unfavorable conditions. New and advanced priming techniques such as nano-particles, gamma-ray, magnetic ray, and UV irradiation and are being developed and applied in many field crops.
Seed priming is the physiological process of controlled seed hydration to enhance sufficient pre- germinative metabolic process, efficient nutrient uptake and water use efficiency, breaking dormancy, timely maturity and crop yield. During imbibition, the water uptake promotes protein synthesis and respiratory activities by using extant messenger ribonucleic acid (mRNA) with the initiation of different physiological activities related to germination. This technology has been found to be the most feasible and economical for uniform seed emergence in most of the field crops. There are many well-developed seed-priming techniques such as hydropriming, osmo-priming, halo-priming, drum- priming, bio-priming, solid matrix priming, whereas the advanced techniques of seed priming include nano- priming, hormonal-priming, magneto-priming. However, priming technology still has several limitations. The prolonged seed treatment during priming may cause loss of seed tolerance to desiccation that reduces seed viability. Similarly, all priming protocols may not lead to significant germination and growth where inappropriate priming conditions may cause degradation in the protective proteins. Hence, extensive research is required in selecting specific priming protocol for different plants regarding germination and growth under various environmental conditions.
LITERATURE CITED
Abou-Zeid, H.M and Moustafa, Y. 2014. Physiological and cytogenetic responses of wheat and barley to silver nanopriming treatment.
Afzal, I., Rehman, H.U., Naveed, M and Basra, S.M.A. 2016. Recent advances in seed enhancements. In New challenges in seed biology-basic and translational research driving seed technology. InTech. 47-74.
Azadi, M.S., Tabatabaei, S.A., Younesi, E., Rostami, M.R and Mombeni, M. 2013. Hormone priming improves germination characteristics and enzyme activity of sorghum seeds (Sorghum bicolor L.) under accelerated aging.
Batool, A., Ziaf, K and Amjad, M. 2015. Effect of halo-priming on germination and vigor index of cabbage (Brassica oleracea var. capitata). Journal of environmental and Agricultural Sciences. 2(7): 1-8.
Basra, S.M.A., Farooq, M., Tabassam, R and Ahmad, N. 2005. Physiological and biochemical aspects of pre- sowing seed treatments in fine rice (Oryza sativa L.). Seed Science Technology. 33(3): 623-628.
Bhardwaj, J., Anand, A and Nagarajan, S. 2012. Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiology and Biochemistry. 57: 67-73.
Bhargava, B., Gupta, Y.C., Dhiman, S.R and Sharma, P. 2015. Effect of seed priming on germination, growth and flowering of snapdragon (Antirrhinum majus L.). National Academy Science Letters. 38: 81-85.
Bujalski, W and Nienow, A.W. 1991. Large-scale osmotic priming of onion seeds: a comparison of different strategies for oxygenation. Scientia Horticulturae. 46(1-2): 13-24.
Bujalski, W., Nienow, A.W and Gray, D. 1989. Establishing the large-scale osmotic priming of onion seeds by using enriched air. Annals of Applied Biology. 115(1): 171-176.
Cantliffe, D.J. 1987. Priming of lettuce for early and uniform emergence under conditions of environmental stress. Acta Horticulturae. 122: 29-38.
Capron, I., Corbineau, F., Dacher, F., Job, C., Côme, D and Job, D. 2000. Sugar beet seed priming: effects of priming conditions on germination, solubilization of 11-S globulin and accumulation of LEA proteins. Seed Science Research. 10(3): 243-254.
Côme, D and Thévenot, C. 1982. Environmental control of embryo dormancy and germination. In: The physiology and biochemistry of seed development, dormancy and germination. 271-298.
Corbineau, F and Côme, D. 2006. Priming: a technique for improving seed quality. Seed testing international. ISTA News Bulletin. 132: 38-40.
Finch-Savage, W.E., Gray, D and Dickson, G.M. 1991. The combined effects of osmotic priming with plant growth regulator and fungicide soaks on the seed quality of five bedding plant species. Seed Science Technology. 19(2): 495-503.
Halmer, P. 2004. Methods to improve seed performance in the field. In: Handbook of seed physiology. 125-65.
Harris, D., Rashid, A., Miraj, G., Arif, M and Yunas, M. 2008. On-farm’ seed priming with zinc in chickpea and wheat in Pakistan. Plant Soil. 306(1-2): 3-10.
Heydecker, W., Higgins, J and Gulliver, R.L. 1973. Accelerated germination by osmotic seed treatment. Nature. 246(5427): 42.
Heydecker, W and Coolbear, P. 1977. Seed treatments for improved performance-survey and attempted prognosis. Seed Science and Technology. 5: 353-425.
Heydecker, W and Gibbins, B.M. 1977. The ‘priming’ of seeds. In: Symposium on seed problems in horticulture. 83: 213-224.
Jie, L., Gong She, L., Dong Mei, O., Fang Fang, L and En Hua, W. 2002. Effect of PEG on germination and active oxygen metabolism in wildrye (Leymu.7 chinensis) seeds. Acta Prataculturae Sinica. 11: 59-64.
Kaur, S., Gupta, A.K and Kaur, N. 2002. Effect of osmo- and hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress. Plant Growth Regulation. 37(1): 17-22.
Kumar, S., Arya, M.C and Sinfh, R. 2010. Management of sweet pepper diseases and growth promotion by Pseudomonas fluorescens and Trichoderma harzianum in mid hills of Central Himalayas, India. Indian Phytopathology. 63(2): 181-186.
Lee, S.S., Kim, J.H., Hong, S.B., Yun, S.H and Park, E.H. 1998. Priming effect of rice seeds on seedling establishment under adverse soil conditions. Korean Journal of Crop Science. 43(3): 194-198.
McDonald, M.B. 2000. Seed priming. Seed technology and its biological basis. Sheffield Academic Press, Sheffield. 287-325.
O’Sullivan, John and Bouw, W.J. 1984. Pepper seed treatment for low-temperature germination. Canadian Journal of Plant Science. 64(2): 387-393.
Passam, H.C., Karavites, P.I., Papandreou, A.A., Thanos, C.A and Georghiou, K. 1989. Osmo-conditioning of seeds in relation to growth and fruit yield of aubergine, pepper, cucumber and melon in unheated greenhouse cultivation. Scientia Horticulturae. 38(3-4): 207-216.
Patanè, C., Cavallaro, V., D’Agosta, G and Cosentino, S.L. 2008. Plant emergence of PEG-osmoprimed seeds under suboptimal temperatures in two cultivars of sweet sorghum differing in seed tannin content. Journal of Agronomy and Crop Science. 194(4): 304-309.
Pozo, M.J and Azcón-Aguilar, C. 2007. Unraveling mycorrhiza-induced resistance. Current Opinion in Plant Biology. 10(4): 393-398.
Reddy, P.P. 2012. Bio-priming of seeds. In: Recent advances in crop protection. Springer, New Delhi. 83-90.
Schwember, A.R and Bradford, K.J. 2005. Drying rates following priming affect temperature sensitivity of germination and longevity of lettuce seeds. Horticultural Science. 40(3): 778-781.
Sliwinska, E and Jendrzejczak, E. 2002. Sugar-beet seed quality and DNA synthesis in the embryo in relation to hydration-dehydration cycles. Seed Science and Technology. 30(3): 597-608.
Taylor,A.G.,Allen, P.S., Bennett, M.A., Bradford, K.J., Burris, J.S and Misra, M.K. 1998. Seed enhancements. Seed Science Research. 8(2): 245-256.
Umair, A., Ali, S., Hayat, R., Ansar, M and Tareen, M.J. 2011. Evaluation of seed priming in mung bean (Vigna radiata) for yield, nodulation and biological nitrogen fixation under rainfed conditions. African Journal of Biotechnology. 10(79): 18122-18129.
Van Nguyen, D., Nguyen, H.M., Le, N.T., Nguyen, K.H., Nguyen, H.T., Le, H.M., Nguyen, A.T., Dinh, N.T.T., Hoang, S.A and Van Ha, C. 2021. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. Journal of Plant Growth Regulation. 1-12.
Varier, A., Vari, A.K and Dadlani, M. 2010. The subcellular basis of seed priming. Current Science. 99: 450-456. Wahid, A., Noreen, A., Basra, S.M., Gelani, S and Farooq, 2008. Priming-induced metabolic changes in sunflower (Helianthus annuus) achenes improve germination and seedling growth. Botanical Studies. 49(4): 343-350.
- Effect of Sowing Window on Nodulation, Yield and Post – Harvest Soil Nutrient Status Under Varied Crop Geometries in Short Duration Pigeonpea (Cajanus Cajan L.)
- Nanotechnology and Its Role in Seed Technology
- Challenges Faced by Agri Startups in Andhra Pradesh
- Constraints of Chcs as Perceived by Farmers in Kurnool District of Andhra Pradesh
- Growth, Yield Attributes and Yield of Fingermillet (Eleusine Coracana L. Gaertn.) as Influenced by Different Levels of Fertilizers and Liquid Biofertilizers
- Consumers’ Buying Behaviour Towards Organic Foods in Retail Outlets of Ananthapuramu City, Andhra Pradesh

