Studies on Sheath Blight Incidence of Rice in Nellore and Chittoor Districts of Andhra Pradesh
0 Views
P. KALAVATHI*, M.K. JYOSTHNA, P. MADHUSUDHAN AND K.V. HARIPRASAD
Department of Plant Pathology, KV.K, Kalikiri, Chittoor-517234.
ABSTRACT
A roving survey was conducted during rabi, 2020-2021 in Nellore and Chittoor districts of Andhra Pradesh to assess the disease severity of sheath blight disease in rice. The per cent disease severity ranging from 6.11 to 13.44 per cent was noticed Maximum disease severity (13.44%) was recorded at Kothapalem village of Nellore district while minimum (6.11%) was recorded at Narasingapuram village of Chittoor district. The maximum disease was observed from tillering stage to harvesting stage.
KEYWORDS: Survey, sheath blight, rice, Rhizoctonia solani.
INTRODUCTION
Rice is the most important staple food crop in the world. Rice being a tropical plant, it can flourish in hot and humid climate. It can be grown in both Kharif & Rabi seasons. Rice is attacked by a number of fungal, bacterial and viral diseases. Among the fungal disease rice sheath blight is regarded as an internationally important disease. Sheath blight is a soil borne disease caused by the fungus Rhizoctonia solani Kuhn AG1-IA. This disease causes significant yield losses about 11.1- 58.0 per cent depending on variety and stage of the crop (Chahal et al., 2003).
Studies on the survey of disease in an area to know the current status of the disease in the various rice growing districts is essential to take decision regarding management of the disease. (Gangopadhyay and Chakrabarti, 1982). In India, this disease was first reported in Punjab, and later in Uttar Pradesh. Further, the disease was reported in Tamil Nadu, Kerala, Andhra Pradesh and Kashmir (Reddy and Reddy, 1986). Disease has been spreaded widely in terms of both occurrence and intensity over the past twelve years. It has become more prevalent on the improved varieties viz., BPT 5204, JGL1798, JGL 384, Swarna, MTU1010, MTU1061 and MTU1075 (Prakasam et al., 2013). The management of sheath blight of rice is to reduce the primary source of inoculum by killing sclerotia or to inhibit their germination. The disease has been efficiently controled by the use of systemic and non-systemic fungicides to seed, soil or foliage applications (Rabindran and Vidhyasekaran, 1996). Because of the hazardous residual effects of chemical fungicides in soil, in recent years several researches have been carried out to assess the potentiality of bio control agents for management of sheath blight, through the application of antifungal bacterial strains isolated from the soil (Nandakumar et al., 2001). Distribution of Bacillus spp. in different ecological habitats and its endospore forming ability, sheath blight disease more possibly controlled by effective strains of B. subtilis among others bio control agents (Qin and Zhang, 2005).
MATERIAL AND METHODS
Roving survey was conducted during Rabi 2020- 21 in major rice growing areas of Chittoor and Nellore districts. In each district, 3 mandals were selected, in each mandals 3 villages were taken. From each village, 3 fields were surveyed to study the disease severity of sheath blight disease.
Four one squire meter quadrants were randomly selected in each field and infected plants were counted in each quadrant based on relative lesions height. The disease severity was calculated based on a scale developed by IRRI, 2002.
Rating scale (based on relative lesion height) 0 – No infection observed
1 -Lesions limited to lower 20% of the plant height 3 – Lesions limited to 20-30% of the plant height
5 – Lesions limited to 31-45% of the plant height 7 – Lesions limited to 46-65% of the plant height
9 – Lesions observed more than 65% of the plant height
Per cent disease index (PDI) was calculated as per the following formula given by Wheeler (1969).
PDI =

Collection of Sheath blight symptoms
During survey characteristics symptoms on the leaf sheath at water level and the lesions in its early stages were circular or oblong with dark brown margin. The lesions were usually confined to the lower leaf sheaths at or near the water level described by Paracer and Chahal (1963). Those diseased samples were collected for isolation of R. solani Kuhn pathogen.
Isolation of pathogen
The causal organism R. solani Kuhn was isolated from the rice plants showing typical sheath blight symptoms under field conditions. Leaf sheath showing typical symptoms was washed in tap water for few minutes and leaf bits of 3-8 mm size were surface sterilized with 1% sodium hypochloride for one minute and then rinsed with sterile distilled water to remove the traces of sodium hypo chlorite. These leaf bits are then transferred to potato dextrose agar medium in petriplates and kept for incubation at 28 ± 2°C. When the growth of the fungus from the leaf bits was seen on the PDA surface, the hyphal bits from the periphery of the culture growing in the petriplates was transferred to the PDA in culture tubes. The culture was purified by hyphal tip method and pure culture was maintained on PDA by regular sub culturing at frequent intervals. Pathogenicity of R. solani was proved by mycelial ball insertion technique as observed by Park et al., (2008) and Nadarajah et al., 2014).
RESULTS AND DISCUSSIONS
The survey data is presented in the table 1. The data indicated that among the all locations surveyed, Nellore district recorded the per cent disease severity ranging from 8.7 to 13.44 while Chittoor district recorded comparatively less disease severity ranging from 6.11 to 12.36 per cent.
In Nellore District, the highest disease severity (13.44%) was recorded in Kothapalem (13.44%) of T.P Gudur (M), whereas the least disease severity (8.7%) was observed in Madharaj gudur of Nellore rural (M).
In Chittoor district, the highest disease severity was recorded in Reddivari palli (12.36%) of Chandragiri (M) whereas the least disease severity was observed in Narasingapuram (6.11%) of Chandragiri (M).
Stage of the crop
During the survey, the disease severity was recorded at different stages of rice crop. In eight villages disease severity was observed during panical intiation stage of the crop, in one village it was during the booting stage, in one village during the dough stage, in four villages it was during the grain filling stage and in four villages during the harvesting stage. Per cent disease severity during the panical intiation stage varied from 6.11 per cent to 13.44 per cent, whereas during the grain filling and harvesting stages it ranged from 8.33 per cent to 13.32 per cent and 8.14 per cent to 12.36 per cent respectively. In Nellore districts surveyed maximum severity was recorded during panical initiation stage and in Chittoor district surveyed maximum severity was recorded during harvesting stage.
In Nellore district, severity ranging from 9.81 per cent (Kothakaluva, Nellore rural (M)) to 13.44 per cent (Kothapalem, T.P gudur (M)) was recorded during the panical initiation stage whereas disease severity during the grain filling stage varied from 8.7 per cent (Madharaj gudur, Nellore rural (M)) to 13.32 per cent (Labur-1, Indhukur peta (M)). Disease severity during the harvesting stage was recorded only in Akuthota (10.35%) of Nellore rural (M), during the dough stage was recorded only in Jagadekapeta (11.43%) and booting stage was recorded only in Narayanareddy peta (9.99%) of Indukur peta (M).
In Chittoor district, panical initiation stage recorded disease severity ranging from 6.11 per cent (Narasingapuram, Chandragiri (M)) to 8.51 per cent (Gajulamadyam, Renigunta (M)). Disease severity observed during the harvesting stage of crop ranged between 8.14 per cent (Athur, Renigunta (M)) and 12.36 per cent (Reddivari palli, Chandragiri (M)) whereas disease severity during grain filling was observed only in Mittapalem (8.33%) of Chandragiri (M).
Crop variety
The per cent disease severity recorded in each variety varied depending upon the place of cultivation. MTU1010 variety was cultivated in six villages had disease severity ranging from 8.14 (Athur, Renigunta (M)) to 13.44 per cent (Kothapalem, T.P gudur (M)), NLR3449 variety was cultivated in three villages had disease severity ranging from 9.81 per cent (Kothakaluva, Nellure rural (M)) to
13.32 per cent (Labur-1, Indukur peta (M)) and ADT 37 variety was cultivated in seven villages disease severity ranged from 6.11 per cent in Narasingapuram village to 12.36 per cent in Reddivaripalli, village of Chandragiri



(M), whereas NLR3354 variety was cultivated only one village Varigunda bit (12.77%)of T.P gudur(M) and NLR3396 variety was cultivated in Akuthota (10.35%) of Nellore rural (M).
In Nellore district, disease sevierity in MTU1010 variety ranged from 8.7 per cent (Madharaj gudur, Nellore rural (M)) to 13.44 per cent (Kothapalem, T.P gudur (M)), NLR3449 variety ranging from 9.81 per cent (Kothakaluva, Nellure rural (M)) to 13.32 per cent (Labur-1, Indukur peta (M)) whereas disease severity in NLR3354 variety was observed only in varigunda bit (12.77%)of T.P gudur(M) and NLR3396 variety in Akuthota (10.35%) of Nellore rural (M).
In Chittoor district, disease sevierity in ADT 37 variety ranged from 6.11 per cent in Narasingapuram village to 12.36 per cent in Reddivaripalli, village of Chandragiri (M) and MTU1010 variety had disease severity 8.14% in Athur, 9.29% in Ammavaripatnam of Renigunta (M).
During survey both Chittoor and Nellore districts clay loam soils were the predominant type of soil for rice cultivated.
Similarly, Reddy et al. (2018) carried out survey for the assessment of sheath blight severity in rice in nine districts of Telangana state. In Adilabad district the maximum severity (9scale) was observed Huzurnagar and Miryalaguda villages. The disease was observed from panicle initiation to grain hardening stage. Whereas some other workers were found different growth stages susceptible for infection. Shahjahan et al. (1990) reported panicle initiation to booting stage is most susceptible stage for sheath blight infection. Pal et al., (2016) also found grain filling stage as most susceptible for sheath blight disease to occur.
Similar results were also recorded by Kapse et al. (2012) and Pal et al. (2015) plant variety is the major factors influencing sheath blight disease. Pratiwi et al. (2021) reported disease severity on rice plants in Northern Sumatra, Indonesia. Highest disease incidence (99.48%) and the highest disease severity (12.38%) was recorded Sumber tani and Talawi in Batubara district.
Isolation of pathogen R. solani
The sheath blight pathogen was isolated from diseased samples collected during the survey and isolated by tissue segment method (Rangaswami and Mahadevan, 1999) Then purified by single hyphal tip method and were identified as R. solani based on morphological characters using the descriptions given by Banniza, 1996.
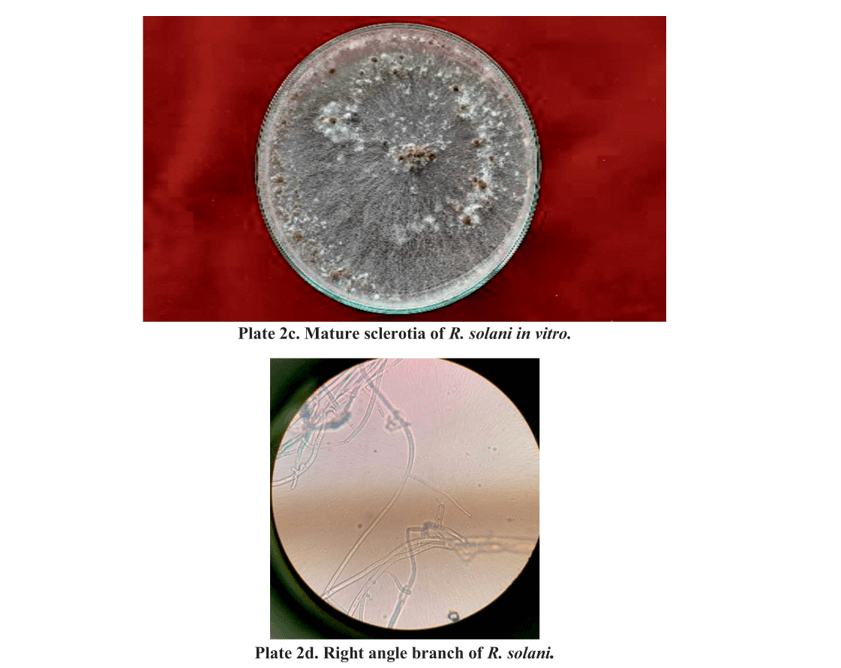
In PDA at 28±1oC the culture was obtained in 3 days of incubation, light brown in colour and occupying the entire Petriplate (Plate 2.2a). Pathogen produced dark brown, irregular, loose type of sclerotial bodies on the PDA (Plate 2.2b, 2.2c). Microscopic examination of the fungal culture revealed broad brown coloured hyphae branching at right angles (Plate 2.2d). These observations were in accordance with Sneh et al., 1991 who described hyphal branching at right angle, constriction at the point of branching of the mycelium and presence of a septum near the branching junction.
A roving survey was conducted during, rabi, 2020-2021 in Chittoor and Nellore districts of Andhra Pradesh to assess the disease severity of sheath blight disease in rice. In Nellore district, highest disease severity (13.44%) was recorded in Kothapalem of T.P Gudur mandal whereas the least disease severity was observed in Narasingapuram (6.11%) of Chandragiri mandal. The per cent disease incidence recorded in each variety varied depending on the place of cultivation disease severity during the panical intiation stage varied from 6.11 to 13.44 per cent, whereas during the grain filling and harvesting stages it ranged from 8.33 to 13.32 per cent and 8.14 to 12.36 per cent respectively. When compared to all the cultivars, maximum disease severity was recorded with MTU-1010 variety at Kothapalem (13.44%) of T.P gudur mandal, Nellore district and ADT 37 variety had recorded minimum disease severity of 6.11% in Narasingapuram of Chandragiri mandal, Chittoor district.
Rice sheath blight pathogen Rhizoctonia solani was isolated from the diseased samples obtained from during survey. Pathogenicity of R. solani was conducted in pots containing rice seedlings at maximum tillering stage by mycelial balls insertion technique.
LITERATURE CITED
Banniza, S., Rutherford, M.A., Bridge, P.D., Holderness, M and Mordue, J.E. 1996. Biological characterization of Rhizoctonia solani in rice-based cropping systems. Proceedings of Brighton Crop Protection Conference. Pests and Diseases. 1: 399-404.
Chahal, S.S., Sokhi, S.S and Ratan, G.S. 2003. Investigations on sheath blight of rice in Punjab. Indian Journal of Plant Pathology. 56: 22-26.
Gangopadhay, S and Chakrabarti, N.K. 1982. Sheath blight on rice. Review of Plant Pathology. 61: 451- 460.
IRRI. 2002. Standard evaluation system for rice: reference guide. International Rice Research Institute, Los Banos.
Kapse, V.V., Bhale, M.S and Jogi, M.J. 2012. Status, distribution and epidemiology of rice diseases in Jabalpur region. Journal of Plant Sciences. 7(1): 185-189.
Nadarajah, K., Omar, N.S., Md. Rosli, M and Tze, O.S. 2014. Molecular characterization and screening for sheath blight resistance using Malasian isolates of Rhizoctonia solani. Hindawi Publishing Corporation Intern BioMed Res. 2014: 1-18.
Nandakumar, R., Babu, S., Viswanathan, R., Raguchander, T and Samiyappan, R., 2001. Induction of systemic resistance in rice against sheath blight disease by Plant Growth Promotining Rhizobacteria. Soil Biology and Biochemistry. 33: 603-612.
Pal, R., Biswas, M.K., Mandal, D., Seni, A and Naik,
B.S. 2015. Prevalence of sheath blight disease of rice in west central table land zone of Odisha. International journal of Bio-Resource Environment and Agricultural Science. 1(3):103-107.
Pal, R., Mandal, D and Biswas, M.K. 2016. Effect of different sowing dates on the development and spread of sheath blight disease in rice. Journal of Crop Weed. 12(1):116-119.
Paracer, C.S and Chahal, D.S. 1963. Sheath blight of rice caused by Rhizoctonia solani Kuhn. A new record in India. Current Science. 32: 328-329.
Park, D.S., Sayler, R.J., Hong, Y.G., Nam, M.H. and Yang, Y. 2008. A method for inoculation and evaluation of rice sheath blight disease. Plant Diseases. 92: 25-29.
Prakasam, V., Ladhalakshmi, D., Laha, G.S., Krishnaveni, D., Sheshu Madhav, M and Jyothi
- 2013. Sheath blight of rice and its management. Technical Bullitin No. 72, Directorate of Rice Research (ICAR), Rajendranagar.
Pratiwi, W., Safni, S., Oemry and Lisnawita. 2021. Distribution of sheath blight disease (Rhizoctonia solani Kuhn) on rice (Oryza sativa L) in Northern Sumatera, Indonesia IOP Conf. Series: Earth and Environmental Science 782 (2021) 042017 International Conference on Agriculture, Environment and Food Security: 2020.
Qin, Z and Zhang, M. 2005. Detection of rice sheath blight for in season disease management using multispectral remote sensing. Journal of Applied Earth Observations and Geo Information. 7:115- 128.
Rabindran, R and Vidyasekaran, P. 1996. Development of a formulation of P. fluorescens PfALR2 for management of rice sheath blight. Journal of Crop Protection. 15(8):715-721.
Rangaswamy, G and Mahadevan A. 1999. Diseases of Crop Plants in India. (4th edition) Prentice Hall of India Pvt. Ltd. New Delhi, 607.
Reddy, D.B., Sagar, V., Prakasam, V and Gajula, S. 2018. Survey on the Sheath Blight disease of Rice in Telangana State. International Journal of Current Microbiology and Applied Sciences. 7: 3525-3531.
Shahjahan, A.K.M., Ahmed, H.U., Sharma, N.R and Miah, S.A. 1990. Epidemiological studies of sheath blight of rice caused by Rhizoctonia solani Kuhn. Bangladesh Journal of Botany. 19(2): 125-133.
Sneh, B., Burpee, L and Ogoshi, A. 1991. Identification of Rhizoctonia species. Annuals of Phytopathological Society. 2:133. Wheeler, B.E.J. 1969. An Introduction to Plant Diseases. John Wiley and Sons Limited. London. P 301.
- Genetic Divergence Studies for Yield and Its Component Traits in Groundnut (Arachis Hypogaea L.)
- Correlation and Path Coefficient Analysis Among Early Clones Of Sugarcane (Saccharum Spp.)
- Character Association and Path Coefficient Analysis in Tomato (Solanum Lycopersicum L.)
- Survey on the Incidence of Sesame Leafhopper and Phyllody in Major Growing Districts of Southern Zone of Andhra Pradesh, India
- Effect of Organic Manures, Chemical and Biofertilizers on Potassium Use Efficiency in Groundnut
- A Study on Growth Pattern of Red Chilli in India and Andhra Pradesh

