Studies on Seed Borne Mycoflora Associated With Groundnut And Their Management (Arachis Hypogea L.)
0 Views
A. VINEETH*, S. VASUNDHARA, B. RUPESH KUMAR REDDY AND P. ARUNA SRI
Department of Seed Science and Technology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
A present investigation was carried to detect seed borne mycoflora in groundnut. A total of three varieties named Nitya haritha, Kadiri lepakshi and TCGS 1694 were collected from Regional Agricultural Research Station (RARS), Tirupati from the farmers participatory seed production programme. The samples collected from these varieties were used to detect seed borne mycoflora by using standard blotter method, agar plating method and rolled paper towel method. A total of seven fungi belonging to four genera viz., A. niger, A. flavus, A. fumigates, A. terreus, Rhizopus sp., M. phaseolina and Penicillium sp. were detected. Agar plating method emerged as the efficient method as it resulted in maximum isolation of seed mycoflora (28.42) followed by next highest (25.33) was recorded in standard blotter method and rolled paper towel method was considered less efficient among these three methods as it recorded (22.08) mean incidence. Aspergillus niger, Rhizopus sp. and Aspergillus flavus found to be predominant.
KEYWORDS: Seed borne pathogens, Groundnut, standard blotter method, agar plating method and Aspergillus flavus.
INTRODUCTION
Groundnut is one of the most significant and commonly grown oil seed crops. Numerous fungi have been shown to be present in groundnut seeds. Storage fungus infects the seeds as they are moved into storage and, in the right circumstances, can quickly spread throughout the bulk. These fungi develop on ground nut seeds; they become visible, can kill the seed, produce an unpleasant odour or flavour, and occasionally the seeds are unfit for human consumption, because the seed fungi produce mycotoxic substance along with a change in the chemical makeup of the seed.
As the groundnut have high content of oil in the seeds and they have low storage period as they are readily subjected to the losses due to the infection from the seed borne pathogens. Hence, we need to have a serious look on controlling these pathogens otherwise the losses due to them are extreme and have great effect on the total production and productivity.
Seed health testing methods such as blotter paper, 2, 4-D blotter, paper towel, agar plate method with PDA, and deep freezing blotter have been utilized to identify internal and external groundnut seed borne mycoflora. Seed samples were collected from various locations and examined for seed-borne fungi to learn about the fungi associated with groundnut seed under local conditions.
MATERIAL AND METHODS
Three genotypes viz, Nityaharitha, Kadiri lepakshi and Vasista (TCGS 1694) were collected from Regional Agricultural Research Station (RARS), Tirupati from the farmers participatory seed production programme. Different seed health testing methods as suggested by ISTA viz., standard blotter method, agar plate method with PDA, paper towel method was employed to detect different seed borne fungi in groundnut seed samples as detailed below.
Standard Blotter Method
Hundred seeds of each sample were tested using standard blotter method in four replications. Three layers of blotter papers of 150 mm diameter were moistened with distilled water and placed in 150 mm sterilized Petri plates. Twenty five seeds were dipped in 1.0 per cent sodium hypochlorite solution and were placed in each Petri plate on the blotters at equal distance. The Petri plates were incubated at 25± 2°C under alternate cycles of 12 h light and 12 h darkness for 7 days in BOD incubator. After incubation, on every third day distilled water was added for the blotter to keep sufficient moisture. After seven days of incubation, the seed was examined under stereoscopic-binocular microscope for associated fungi.
Agar Plate Method with Potato Dextrose Agar
Hundred seeds of each sample were surface sterilized and four replications of 25 seeds were placed in Petri plates of 150 mm diameter, containing 50 ml potato dextrose agar. The Petri plates were incubated as described under standard blotter method. After seven days of incubation, the fungal growth on seed was examined under stereoscopic binocular microscope.
Paper Towel Method (Rolled Paper Towel Method)
Hundred seeds of each sample in four replicates were surface sterilized before placing on two layers of moist germination paper at the rate of ten seeds per row in ten rows, which were then placed on a polythene paper and rolled carefully to avoid any excess pressure on seed. These paper towels in four replications were incubated under ambient conditions. After ten days of incubation, the fungal growth on seed was examined under stereoscopic binocular microscope.
RESULTS AND DISCUSSION
Standard blotter method
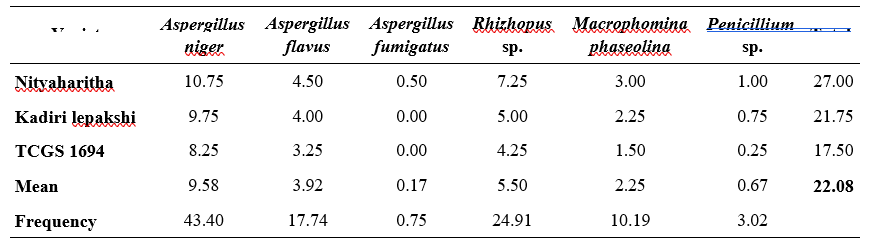
By using standard blotter method various fungi were isolated from the seed samples. A total of seven fungi belonging to four genera viz., A. niger, A. flavus fumigates, A. terreus, Rhizopus spp., M. phaseolina and Penicillium spp. were isolated in all the samples irrespective of variety (Table 1).
From seven fungal species, A. niger was found to be predominant (39.14%) followed by Rhizopus (23.03%) and A. flavus (18.09%) while A. terreus was found to be least occurring (2.30%). The per cent incidence of seed borne pathogens ranged from 21 to 31. The total per cent incidence of seed borne pathogens was highest (31) in Nityaharitha variety. The lowest per cent incidence 21 was found in TCGS 1694. Kadiri lepakshi recorded (24) intermediate per cent incidence of seed borne pathogens among the three varieties Per cent incidence of A. niger ranged from 8.75 to 11.25 followed by Rhizopus spp. (4.5 to 7.75) and the least occurring pathogen was A. terreus (0.25 to 1.25). In average from all three varieties A. niger is occupied the mean highest incidence of (9.92) A. nniger is followed by Rhizopus spp. (5.83), A. flavus (4.58) and the lowest average (0.58) was recorded in A. terreus.
Similar results were obtained by Ingle (2020) using standard blotter method where Aspergillus spp. was predominant among the fungi identified in groundnut crop. Suhendar (2023) and Aslam (2015) also identified Aspergillus spp., Penicillium spp., Rhizopus spp. by using seed detection methods in rice, maize, sorghum, groundnut and cowpea crops.
Agar plate method with PDA
A total of seven fungal species belonging to four genera were isolated using agar plate method with PDA (Table 2). Similar to standard blotter method, agar plate method also recorded the highest occurrence frequency of A. niger (37.83) followed by Rhizopus spp (21.41) and A. flavus (18.18). A. fumigates recorded the least frequency (2.64). Other fungal species isolated were terreus (3.81), M. phaseolina (10.85) and Penicillium (5.28).
The total per cent incidence of mycoflora ranged from 24 to 34.5. The per cent incidence of A. niger ranged from 9.25 in TCGS 1694 to 12.75 in Nityaharitha variety while A. flavus ranged from 4.50 in TCGS 1694 to 6.25. The per cent incidence of A. fumigatus ranged from 0.50 in TCGS 1694 to 1.25 in Nityaharitha. In case of terreus both Kadiri lepakshi and TCGS 1694 had same incidence (1%). Surprisingly incidence of A. terreus increased from 2.30 in blotter method to 3.81 in case of agar method. The lowest incidence in standard blotter method was seen in A. terreus and the A. fumigates in PDA method.
The mean per cent occurrence was highest (10.75) in A. niger followed by Rhizopus spp. (6.08) and least mean per cent was recorded in A. fumigates (0.75). In addition to these, other fungi recorded are A. flavus (5.17), M. phaseolina (3.08), Penicillium spp. (1.50) and terreus (1.08).
Variety Nityaharitha was found to be more infected with a total incidence of 34.5% and TCGS 1694 was found to be least infected with total incidence of 24%. Kadiri lepakshi was stayed intermediate with a total incidence of (26.75). The results are in accordance with Jogdand and Talekar (2010) and Mohammed and Chala (2014) where the presence of A. niger, A. flavus, Rhizopus, Fusarium, and Penicillium in groundnut seed were observed using the agar plate method. Aspergillus spp, Fusarium spp, Alternaria, Macrophomina, and Penicillium spp were identified as major seed-borne fungi in groundnut seed
Table 1. Detection of seed borne mycoflora in groundnut varieties using standard blotter method
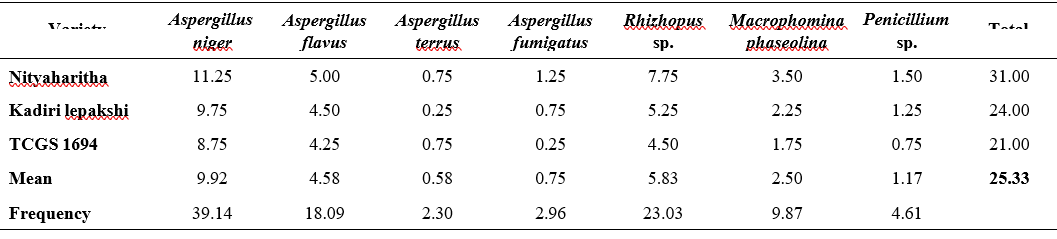
Table 2. Detection of seed borne mycoflora in groundnut varieties using agar plate method with PDA
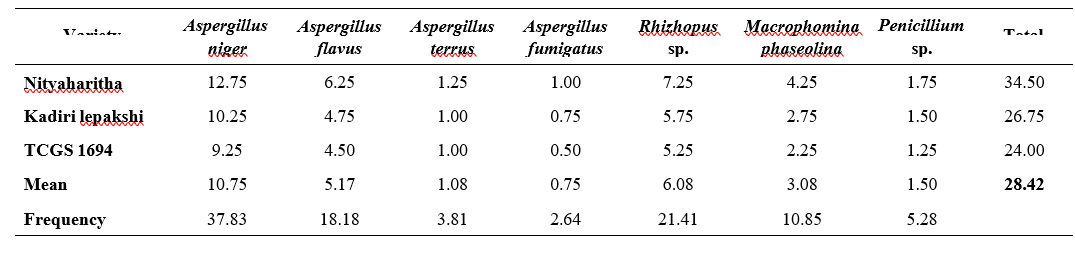
Table 3. Detection of seed borne mycoflora in groundnut varieties using rolled paper towel method
by Ingle (2020). Al-Amodi (2016) also found abundant niger, A. flavus, M. phaseolina, and R. stolonifer on groundnut seed.
Rolled paper towel method
Using the rolled paper towel method, six fungal species from four genera were isolated. All six fungal species were isolated from all samples, irrespective of variety (Table 4.3).
The per cent frequency of pathogen incidence was highest (43.40) in A. niger followed by Rhizopus spp (24.91) and A. flavus (17.74). A. fumigates (0.75) was the least followed by Penicillium spp (3.02%). Other fungus isolated from these farmers’ saved samples was phaseolina (10.19%).
The total per cent incidence of mycoflora ranged from 17.5 in TCGS 1694 to 27 in Nityaharitha. The highest per cent incidence of A. niger (10.75) was found in Nityaharitha. A. fumigatus was only found in variety Nityaharitha and no incidence of this pathogen was observed in other varieties.
The mean occurrence per cent of A. niger was highest (9.58) followed by Rhizopus spp (5.50) and least in A. fumigatus (0.17). In addition to these, others were recorded the averages of A. flavus (3.92), M. phaseolina (2.25) and Penicillium spp. (0.67). Patil (2018) obtained similar results that he identified important fungi such as Aspergillus spp. and Fusarium spp.
The three methods showed difference in the obtained results although in common a total of seven fungi belonging to four genera viz., A. niger, A. flavus, fumigates, A. terreus, Rhizopus sp., M. phaseolina and Penicillium sp. were isolated in all the samples irrespective of variety. Nityaharitha recorded highest per cent incidence of seed mycoflora followed by Kadiri lepakshi and least incidence was recorded in TCGS 1694 irrespective of the methods.
ACKNOWLEDGEMENT
The authors thank Acharya N.G. Ranga Agricultural University for providing financial assistance and support in the conduct of experiment at Department of Seed Science and Technology, S. V. Agricultural College, Tirupati, A.P.
LITERATURE CITED
Al-Amodi, M.O. 2016. Fungi associated with seeds of Ashford variety of groundnut grown in Yemen and its disinfection in vitro using Sodium Hypochlorite. Journal of Global Biosciences. 5(1): 3414-3422.
Aslam, M.F., Irshad, G., Naz, F., Aslam, M.N and Ahmed, R. 2015. Effect of seed-borne mycoflora on germination and fatty acid profile of peanuts. Pakistan Journal of Phytopathology. 27(2): 131- 138.
Ingle, S.S., 2021. Assessment of ground nut seed mycoflora. European Journal of Molecular & Clinical Medicine. 7(9): 2766-2768.
ISTA 1966. International rules for seed testing. Proc. Int. Seed Asso. 32: 565-589.
Jogdand, S.K and Talekar, S.M. 2010. Fungal population on seeds of Arachis hypogea L. Journal of Ecobiotechnology. 2(6): 11-13.
Mohammed, A and Chala, A. 2014. Incidence of Aspergillus contamination of groundnut (Arachis hypogaea L.) in Eastern Ethiopia. African Journal of Microbiology Research. 8(8): 759-765.
Patil, A.C., Suryawanshi, A.P., Anbhule, K.A., Raner, R.B and Hurule, S.S. 2018 Detection of sunflower seedborne mycoflora and their effect on seed and seedling parameters. International Journal of Current Microbiology and Applied Sciences. 6: 2509-2514.
Suhendar, M.A., Koswanudin, D., Hidayatun, N., Diantina, S., Manzila, I. and Zulchi, P.T. 2023, April. Detection of pathogens to test seed health of some food crops in storage. In IOP Conference Series: Earth and Environmental Science (Vol. 1160, No. 1, p. 012041). IOP Publishing.
- Genetic Divergence Studies for Yield and Its Component Traits in Groundnut (Arachis Hypogaea L.)
- Correlation and Path Coefficient Analysis Among Early Clones Of Sugarcane (Saccharum Spp.)
- Character Association and Path Coefficient Analysis in Tomato (Solanum Lycopersicum L.)
- Survey on the Incidence of Sesame Leafhopper and Phyllody in Major Growing Districts of Southern Zone of Andhra Pradesh, India
- Effect of Organic Manures, Chemical and Biofertilizers on Potassium Use Efficiency in Groundnut
- A Study on Growth Pattern of Red Chilli in India and Andhra Pradesh

