Screening of Black Gram, Vigna Mungo L. Genotypes Against Spotted Pod Borer, Maruca Vitrata (Geyer)
0 Views
B. DEBASREE*, M. RAJASRI, K. DEVAKI AND D. MOHAN REDDY
Department of Entomology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
Field screening of forty seven black gram (Vigna mungo L.) genotypes against spotted pod borer, Maruca vitrata was carried out during late rabi, 2023-24 under field conditions at S.V. Agricultural College, Tirupati, Andhra Pradesh. The genotypes were categorized based on the per cent pod damage and were converted to Pest Resistance Rating (PRR) scale for all the genotypes. Among the 47 genotypes, 17 genotypes exhibited highly resistant reaction with PRR scale 2, whereas, 19 genotypes recorded resistance to pod borer damage. Nine genotypes were categorized as moderately resistant and one genotype was recorded as tolerant. The maximum pod damage was found in LBG 961 (8.24%) and significantly least pod damage was recorded in GBG 269 (0.57%).
KEYWORDS: Blackgram, Screening, Maruca vitrata, Pest Resistance Rating, Pod damage.
INTRODUCTION
Pulses are nutrient rich edible seeds of legumes commonly known as grain legumes. Due to their high nutritional value and low price, they are referred as “poor man’s meat”. The productivity of black gram is constrained by various biotic and abiotic stresses and insect pests and diseases rank among the biotic stresses having a significant impact on crop output and productivity. Among them, the spotted pod borer, Maruca vitrata is a serious pest of pulses, causing substantial damage to flowers and pods by webbing them and feeding from the inside. This hiding behaviour of the pod borer protects the larvae from both biotic and abiotic stresses and also makes it difficult to manage the insect with contact insecticides. Continuous and indiscriminate use of chemical insecticides, besides creating health hazards to human and animal life, has led to the development of resistance and the destruction of natural enemies as well as creating environmental pollution. To mitigate or minimize chemical pesticide usage, it is more desirable to use alternate practices. Host plant resistance can play a pivotal role in pest management of pulse crops and resistance to insect pests should be one of the major criteria in the development of lines with insect resistance and through resistance breeding programmes release of new varieties. In this study, 47 black gram genotypes were screened against M. vitrata under field conditions and their level of resistance were categorized based on Pest Resistance Rating (PRR).
MATERIAL AND METHODS
Field experiment was conducted during late rabi, 2023-24 at S.V. Agricultural College, Tirupati, Andhra Pradesh. The experiment was conducted in Randomized Block Design (RBD) with two replications. A total of 47 varieties were sown with each variety in a single row of 8 m length with 60 cm × 10 cm spacing. Standard agronomic practices were followed as per ANGRAU package of practices without any insecticidal applications. The data was recorded on larval population and per cent pod damage by M. vitrata on different black gram genotypes. For recording observations, five plants were randomly selected and number of larvae present on each plant were recorded. Pod damage at maturity of the crop was assessed based on number of damaged pods out of total pods from five randomly selected plants. The per cent pod damage was determined by subjecting the data to the following formula.
![]()
Categorization of black gram genotypes
Black gram genotypes were categorized based on per cent pod damage and Pest Resistance Rating (PRR) (1-9 scale). The per cent pod damage at maturity of test entry is compared with that of the susceptible check in the trial and the pest resistance (per cent) was calculated by using a formula (Abbott, 1925).
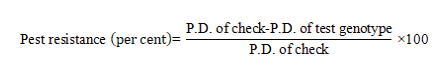
where, P.D. = Mean of per cent pods damaged
The pest resistance percentage is then converted to 1 to 9 scale based on the following table:
RESULTS AND DISCUSSION
Larval population
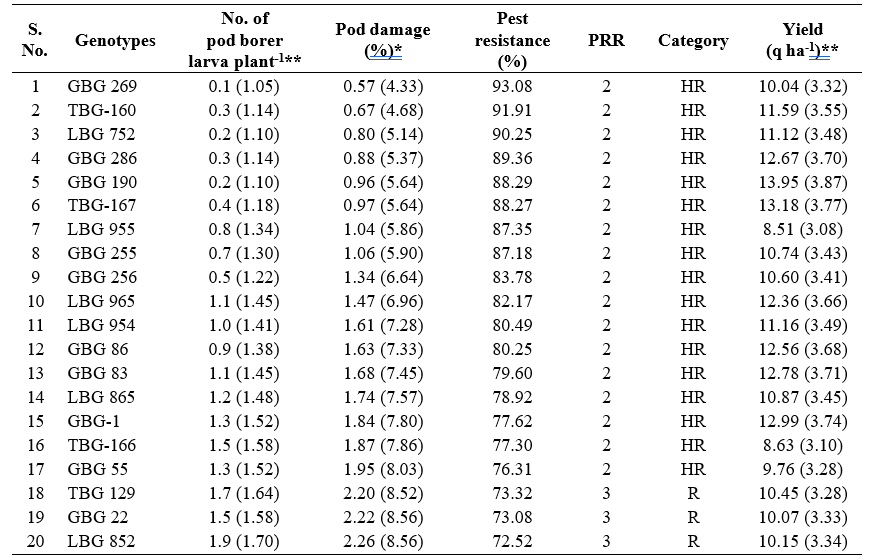
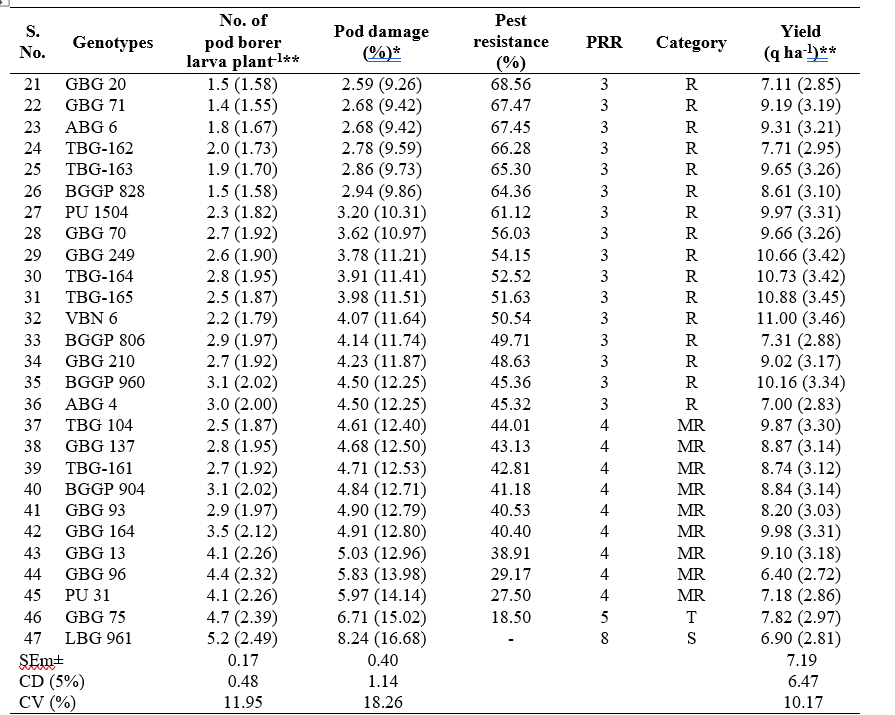
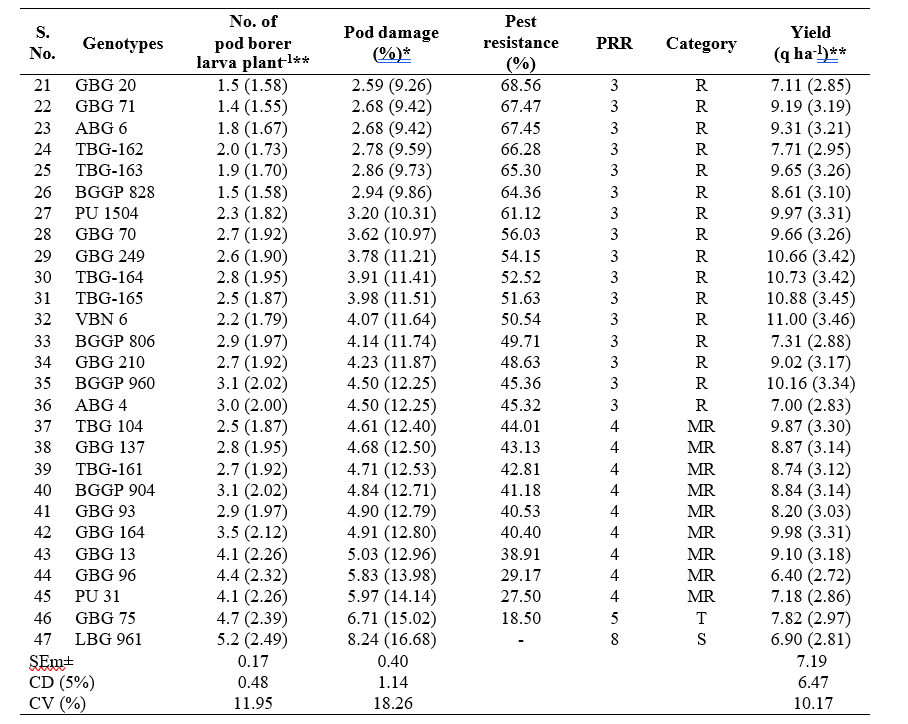
The infestation of M. vitrata varied from 0.1 to
5.2 larva plant-1 in different black gram genotypes. The infestation of the pest was lowest in genotype GBG 269 (0.1 larva plant-1) followed by LBG 752 (0.2 larva plant-1) and GBG 190 (0.2 larva plant-1) were on par with each other. Significantly highest population was recorded on LBG 961 (5.2 larva plant-1) followed by GBG 75 (4.7 larva plant-1) and GBG 96 (4.4 larva plant-1). The larval population recorded in other entries ranged from 0.3 to
4.1 larva plant-1 (Table 2).
The findings of Chauhan et al. (2021) support the present findings who reported that the least incidence of larvae was observed in NDMK 15-513 (1.12 larvae per five plants) and least pod damage in IPM 14-7 (1.33%).
Pod Damage
The per cent pod damage by Maruca vitrata ranged between 0.57 to 8.24 per cent during late rabi, 2023- 24 Among the 47 black gram genotypes evaluated, significantly lowest per cent pod damage of 0.57 per cent was recorded with GBG 269 followed by TBG-160, LBG 752, GBG 286, GBG 190, TBG-167, LBG 955 and GBG 255 with less than 1per cent pod damage. LBG 961 recorded the highest per cent pod damage (8.24%) followed by GBG 75 (6.71%) and PU 31 (5.97%) (Table 2).
The present findings are in agreement with Soundararajan and Chitra (2014) who reported that pod damage due to M. vitrata was in the range of 0 to
14.0 per cent among different genotypes. Among the all genotypes, minimum and maximum pod damage were recorded in genotypes CBG 08-008 and CBG 08-037, respectively. Similarly, Chandekar et al. (2022) reported
7.5 per cent pod damage in PU 31 due to the infestation of M. vitrata. Kumar and Singh (2018) observed 15.40 per cent and 16.33 per cent pod damage in PU 31.
Categorization of black gram genotypes based on their reaction to per cent pod damage by spotted pod borer, M. vitrata and pest resistance rating scale.
Among these 47 black gram genotypes, 17 genotypes viz., GBG 269, TBG-160, LBG 752, GBG 286, GBG 190, TBG-167, LBG 955, GBG 255, GBG 256, LBG 965, LBG 954, GBG 86, GBG 83, LBG 865, GBG-1, TBG-166 and GBG 55 were categorised as highly resistant (HR) based on pest resistance rating scale 2, whereas, 19 genotypes were found to be resistant (R) with PRR scale 3. Nine genotypes with PRR scale 4 were found to be moderately resistant (MR). Only one genotype, GBG 75 was found to be tolerant (T) with PRR scale 5 and the genotype, LBG 961 was found to be susceptible with PRR scale 8 and highest per cent pod damage (8.24%) with respect to M. vitrata in black gram (Table 2).
The present results were also supported by Naik and Mallapur (2019) who have screened and reported that PU 31 was resistant to the pod borer, M. vitrata damage. However, in the present investigation, PU 31 showed a moderately resistant reaction in terms of pod damage by M. vitrata. The difference in resistant reaction observed in PU 31 could be due to the local

Table 1. Categorization of black gram genotypes based on Pest Resistance Rating (PRR) scale (1-9)

Table 2. Screening of black gram genotypes against spotted pod borer, M. vitrata under field conditions during late rabi, 2023-24.
environmental conditions prevailing during the period of experimentation. Yadav et al. (2021) screened 15 black gram genotypes against pod borers i.e. M. vitrata and Helicoverpa armigera and reported that KU-99-05 and Azad Urd-1 were found resistant with minimum pod infestation of 7.67 and 9.67 per cent, respectively. Kumar and Singh (2018) reported that the maximum population of spotted pod borer was observed in genotypes CO5 followed by VBN 4 and Azad 4 and the least population was observed with genotypes IPU 94-1, IPU 7–3 and WBC-108 and PU-40. The maximum pod damage was observed with the genotype VBN 4 followed by CO 5 and LBG 20 and the least damage with IPU 94-1 and IPU 2–43. Sandhya Rani et al. (2014) reported that five genotypes, KM-9-128 (3.5%), KM-9-136 (5.8%), RMG-492 (8.34%), LGG-527 (9.5%) and LGG-538 (10.0%) were found to be tolerant to pod borers.
Impact of M. vitrata pod damage on yield in different black gram genotypes.
Results revealed that yield in different black gram genotypes ranged from 6.40 to 13.95 q ha-1. GBG 190 recorded the highest yield of 13.95 q ha-1 followed by TBG-167 (13.18 q ha-1) and GBG-1 (12.99 q ha-1). The GBG 96 recorded the lowest yield of 6.40 q ha-1 followed by LBG 961 (6.90 q ha-1) and ABG 4 (7.00 q ha-1) (Table 2).
These findings are in agreement with Singh and Srivastava (2017) who reported that the highest grain yield was recorded from VGG 10-008 (819 kg ha-1) while the lowest grain yield was reported from KM 2348 (416 kg ha-1). Similarly, Kumar and Singh (2014) reported that the highest yield was obtained from RVSU- 11-8 (7.82 qha-1), followed by KPU-1-10 (7.51 q ha-1) and AKU10-4 (6.87 q ha-1), and the lowest yield from TU-631 (2.33 q ha-1).
LITERATURE CITED
Abbott W.S. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 18: 265- 267.
Chandekar, P., Singh, V and Katlam, B.P. 2022. Screening of different germplasm of urdbean against pod borer complex. Journal of Current Research in Food Science. 3(2): 94-97.
Chauhan, R., Singh, A.K and Premkumari, S. 2021. Screening of Mung Bean Germplasm Against Pod Borer Complex. Indian Journal of Entomology. 83(2): 264-265.
Kumar, M and Singh P.S. 2014. Screening of black gram genotypes against major insect pests. Indian Journal of Entomology. 76(1):84-86.
Kumar, M and Singh, P.S. 2018. Screening of blackgram genotypes against spotted pod borer. Indian Journal of Entomology. 80 (4): 1513-1515.
Naik, M.G and Mallapur, C.P. 2019. Field screening of blackgram genotypes against spotted pod borer, Maruca vitrata (Geyer). Journal of Entomology and Zoology Studies. 7 (3): 631-634.
Sandhya Rani, C., Rao, G.R., Chalam, M.S.V., Kumar, P.A and Rao, V.S. 2014. Field screening of greengram genotypes against Maruca vitrata in Summer. Journal of Agricultural, Biological and Environmental Sciences. 1: 53-60.
Singh, S and Srivastava, C.P. 2017. Field screening of some green gram [Vigna radiata (L.) Wilczek] genotypes against spotted pod borer, Maruca vitrata (Fabricius). Journal of Entomology and Zoology Studies. 5 (4): 1161-1165.
Soundararajan, R.P and Chitra, N. 2014. Field screening of black gram, Vigna mungo L. germplasm for resistance against pod borer complex. Indian Journal of Entomology. 76 (2): 142-148.
Yadav, A., Singh, G., Yadav, A., Singh, H., Singh, V and Singh, P. 2021. Screening of black gram genotypes against major pod borers. Legume Research-An International Journal. LR-4686.
- Effect of Sowing Window on Nodulation, Yield and Post – Harvest Soil Nutrient Status Under Varied Crop Geometries in Short Duration Pigeonpea (Cajanus Cajan L.)
- Nanotechnology and Its Role in Seed Technology
- Challenges Faced by Agri Startups in Andhra Pradesh
- Constraints of Chcs as Perceived by Farmers in Kurnool District of Andhra Pradesh
- Growth, Yield Attributes and Yield of Fingermillet (Eleusine Coracana L. Gaertn.) as Influenced by Different Levels of Fertilizers and Liquid Biofertilizers
- Consumers’ Buying Behaviour Towards Organic Foods in Retail Outlets of Ananthapuramu City, Andhra Pradesh

