Morphological Characterization of Native Isolates Of Beauveria Bassiana (Balsamo) Vuillemin and Metarhizium Anisoplieae (Metchnikoff) Sorokin From Rayalaseema Region of Andhra Pradesh
0 Views
S. ABDUL MUJEEB*, K. MANJULA, P.N. HARATHI, A. KANDAN AND P. LAVANYA KUMARI
Department of Entomology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
This study characterizes the morphological traits of native isolates of Beauveria bassiana and Metarhizium anisopliae, two key entomopathogenic fungi (EPF) used in biocontrol of agricultural pests. In the present study conducted at S.V. Agricultural College,Tirupati, native isolates of B. bassiana and M. anisopliae were collected from major field crops like groundnut and maize from major groundnut growing districts of Rayalaseema region. These isolates were cultured on SMAY media and morphological characters were studied. Morphological characters viz., width of mycelium, width of conidiophore and length and width of conidia were measured. Observations revealed that B. bassiana isolates exhibited cottony white mycelium with globose conidia, while M. anisopliae isolates developed greenish colonies with elongated, cylindrical conidia. Conidia structure varied between the isolates, highlighting intraspecific diversity. In B. bassiana conidia were measured from 1.9 – 3.1 μm in length and 1.6 – 2.8 μm in width whereas in case of M. anisopliae, conidia were measured from 6.7 to 7.2 μm in length and 3.2 to 3.5 μm in width..
KEYWORDS: Beauveria bassiana, Metarhizium anisopliae, native isolates, conidiophore, conidia.
INTRODUCTION
The use of Entomopathogenic fungi (EPF) like Beauveria bassiana and Metarhizium anisopliae is upsurging in recent years for the management of crop insect pests. EPF are considered better than synthetic insecticides as they are safe for humans, sustainable to the environment, target-specific in nature and more commonly in natural occurrence.
bassiana, a filamentous fungus, belongs to a class of insect pathogenic deuteromycete and strains of
bassiana are highly adapted to particular host insects. A broad range of B. bassiana has been isolated from a variety of insects worldwide which are of agricultural importance. B. bassiana is a fungus that grows naturally in soils throughout the world and it has a wide host range that causes white muscardine disease among the insects of orders Lepidoptera, Coleoptera, Orthoptera, Diptera, Dermaptera, hymenoptera and Hemiptera (Toledo et al., 2008).
M. anisopliae is a mitosporic fungus with asexual reproduction, which belongs to Deuteromycetes. Many attempts have been made to use these naturally occurring fungi against insect pests including soil-inhabiting pests like scarab larvae. However use of entomopathogenic fungi for controlling insect pests did not resurface until the 1970s as public awareness of pesticide hazards increased. During 2018-20, it is observed to cause epizootics in Spodoptera litura and Aproaerema modicella in rabi and kharif groundnut respectively in Tirupati region (Manjula et al., 2019).
Morphological and molecular identification of fungi is considered as essential step in the selection of biocontrol agents (Boucias et al., 2000). Diversity within entomopathogenic fungal species has been traditionally analysed using morphological features by assessing the phenotypic characteristics. However, assessing the species diversity by analysing morphological features alone could lead to ambiguity with regard to identification at species level, due to the observed divergence of morphological characters produced by genetic variability. Therefore applications of molecular methods have been quite useful to detect the level of variation among species (Fernandes et al., 2009; Nishi et al., 2011).
In recent years, these two prominent fungi have been infecting various lepidopteran insect pests in the Rayalaseema region and other parts of Andhra Pradesh.
Keeping the importance of these two fungal pathogens in view, native isolates collected from different areas within the Rayalaseema region were morphologically characterized.
MATERIAL AND METHODS
Purification of fungal isolates
Roving survey was conducted for collection of lepidopteran caterpillars infected with two entomopathogenic fungi showing Beauveria and Metarhizium growth from field crops like Groundnut and maize during kharif, 2022 and rabi, 2022-23. Soil samples were also collected from the surveyed locations. The infected cadavers were inoculated onto SMAY (Saboraud’s maltose Agar Yeast) medium and the soil samples were serially diluted upto 10-4 and transferred 1ml of suspension onto the medium containing petriplates (Meyling and Nicolai, 2007). The inoculated plates were maintained in BOD at 22°C. Growth of fungi was monitored for 10 days and obtained pure cultures were maintained in the laboratory.
Morphological Characterization of Isolates of bassiana and M. anisopliae
Morphology characters were studied at Insect pathology laboratory, Department of Entomology and Central instrumentation laboratory at S. V. Agricultural College, Tirupati.
The characters of mycelium, conidiophores and conidia of fungal isolates were studied. In the laminar airflow chamber, with the help of a sterilized inoculating needle, took a minute portion of B. bassiana culture from the Petri plate and placed it on the sterilized glass slide. Then a drop of lactophenol cotton blue was placed. The fungus was spread with help of a needle, placed a cover slip and removed excess lacto-phenol cotton blue with blotting paper. The measurements viz.,width of mycelium, width of conidiophore as well as length and width of conidia were studied with the help of Research microscope. In the same way, morphological identification was carried out for M. anisopliae isolates also.
RESULTS AND DISCUSSION
A total of six isolates of B. bassiana and six isolates of M. anisopliae were obtained from the survey. The isolates were named by taking into consideration of the village and district name. They were named as followed.
- B. bassiana isolate Bb. Dpl. Ctr (Dandapalle, Chittoor)
- B. bassiana isolate Bb. Pmr. Ctr (Palamaner, Chittoor)
- B. bassiana isolate Bb. Pkd. knl (Pattikonda, Kurnool)
- B. bassiana isolate Bb. Dvk. Knl (Devanakonda, Kurnool)
- B. bassiana isolate Bb. Gty. Atp (Gooty, Anantapuramu)
- B. bassiana isolate Bb. Utr. Kdp (Utukur, Kadapa)
- M. anisopliae isolate Ma. Dpl. Ctr (Dandapalle, Chittoor)
- M. anisopliae isolate Ma. Pmr. Ctr (Palamaner, Chittoor)
- M. anisopliae isolate Ma. Pkd. Knl (Pattikonda, Kurnool)
- M. anisopliae isolate Ma. Dvk. Knl (Devanakonda, Kurnool)
- M. anisopliae isolate Ma. Gty. Atp (Gooty, Anantapuramu)
- M. Anisopliae isolate Ma. Rkl. Atp (Rekulakunta, Anantapuramu)
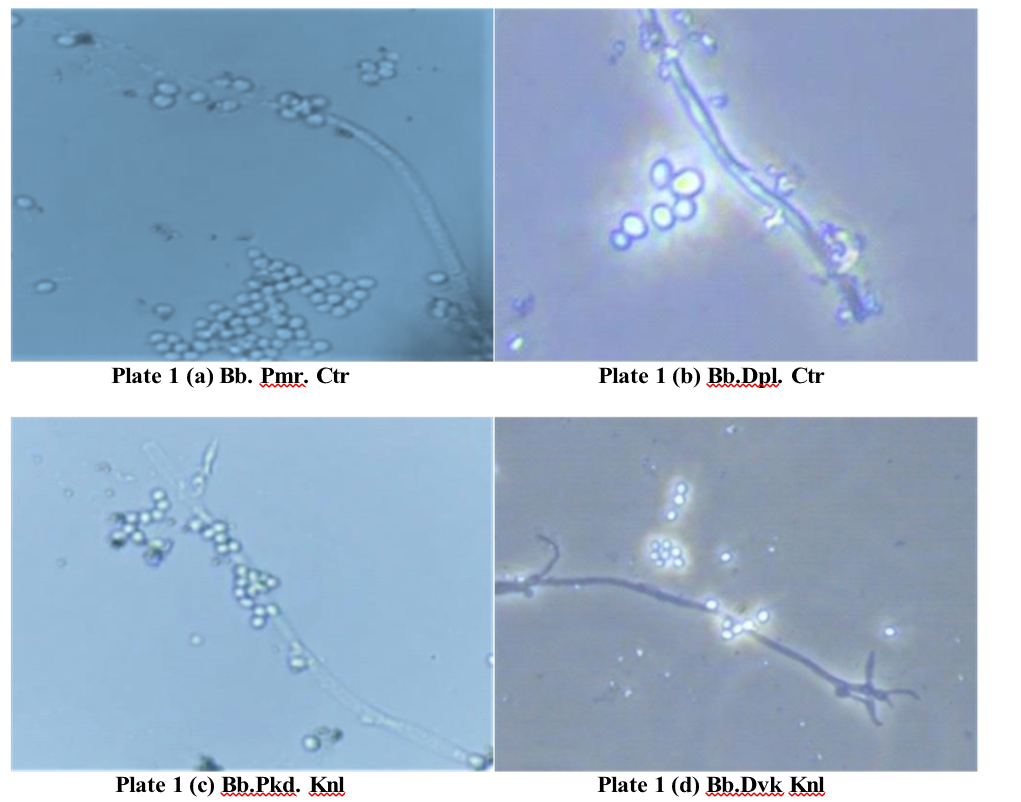
Morphological characters of B. bassiana isolates
The beginning of mycelial development in case of
bassiana isolates was noticed as white tiny spots two days after inoculation of the isolates on SDAY medium. Then, day by day, there was a noticeable increase in the formation of mycelia, starting as a white cottony colony and gradually turning from white to light yellow, becoming powdery during sporulation. Reverse, pale yellow with white margin. It took about 7-8 days to cover whole petriplates with mycelia development. After 10 days, white powdery spores were observed on the mycelium. Microscopic examination of mycelia threads of fungi indicated that they are hyaline and Mycelium (hypha) is septate, branched, hyaline, smooth walled, 1.2–2.3 µm wide.
Isolate 1. Beauverai bassiana, Palamaner, Chittoor (Bb. Pmr. Ctr )
The fungal development begins with the growth of white, cottony mycelium on the SDAY medium, with
hyphae measuring 1.3-2.2 μm in width. Simultaneously, measuring 3.1-5.1 µm in width, and featured an apex sporulation occurs as conidiophores develop, typically with an indeterminate denticulate rachis. In the later solitary but often clustering in groups of two or more. stages, conidia were produced in clusters, displaying a These conidiophores have a sub-spherical to ampulliform round to oval shape (Plate 1c). The conidia measured base, 3.5–5.2 μm wide, and extend into an indeterminate between 2.1-2.9 μm in length and 1.9-2.5 μm in width. denticulate rachis at the apex. Conidia are produced in clusters, globose or subglobose in shape, and eventually mature to a size of 2.5–3.1 μm in length and 2.1–2.8 μm in width (Plate 1a).
Isolate 2. Beauveria bassiana, Dandapalle, Chittoor (Bb. Dpl. Ctr )
On the SDAY medium white mycelial growth was observed after two days of inoculation. As the to growth progressed, puffy white mycelium developed, accompanie db ysimultaneou ssporulation .Th emycelium was branched, hyaline, and septate, with a width ranging from 1.2 – 2.1 μm. In the next stage, conidiophores began to form in clusters of three or more, each with a spherical
base measuring 3.2–4.9 µm in width. As the fungus matured, conidia were produced in clusters, ranging from oval to globose in shape (Plate 1b). These conidia measured 2.3 – 3.0 μm in length and 2.0-2.7 μm in width.
Isolate 3. B. bassiana. Pattikonda, Kurnool (Bb. Pkd. Knl )
During the initial stages of fungal development on SDAY medium, a white puffy growth was observed, accompanied by simultaneous sporulation of the fungal hyphae. The developing mycelium was septate, hyaline, and branched, with a width ranging from 1.0-2.1 μm. As the fungus advanced, conidiophores began to form, typically appearing in clusters of two or more.
, measuring 3.1-5.1 µm in width, and featured an apex with an indeterminate denticulate rachis. In the later stages, conidia were produced in clusters, displaying a round to oval shape (Plate 1c). The conidia measured between 2.1-2.9 μm in length and 1.9-2.5 μm in width.
Isolate 4. B. bassiana. Devanakonda, Kurnool (Bb. Dvk. Knl )
On SDAY medium, white mycelial growth was observed within two days of inoculation, characterized by a cottony white appearance and simultaneous sporulation. As development progressed, the mycelium. which was branched, hyaline, and septate, was measured betwee n1.2 -2. 0μ mi nwidth. Conidiophore sformed clusters of two or more, featuring a sub-spherical base with a width of 2.9 – 5.0 µm. As the fungus matured, conidia were produced in clusters, ranging in shape from oval to globose (Plate 1d). These conidia were measured at 2.2-2.8 μm in length and 2.0-2.6 μm in width.
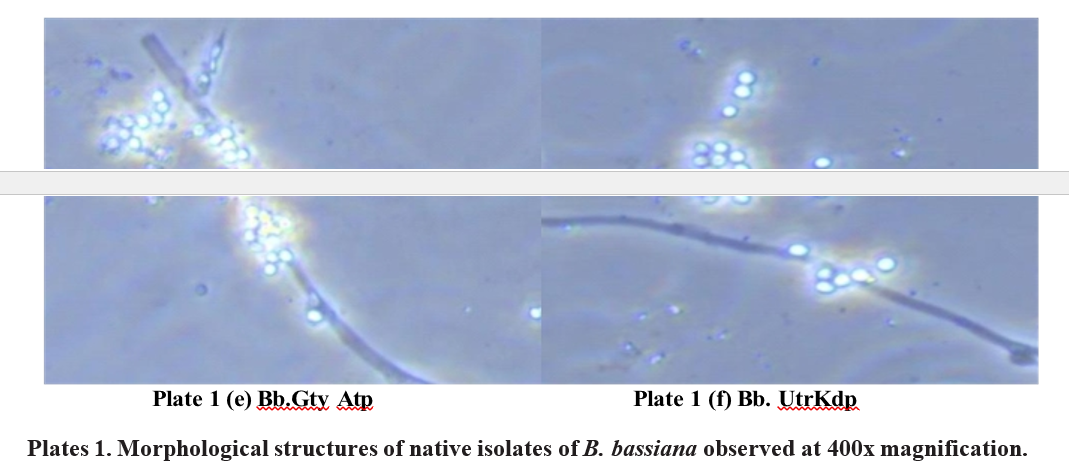
Isolate 5. B. bassiana. Gooty, Anantapuramu (Bb. Gty. Atp )
In the initial stages of fungal development on SDAY medium, the puffy growth of mycelia with abundant sporulation of B. bassiana was observed in the petri plate. The mycelia, ranged from 1.0-2.0 μm in width. As development continued, straight conidiophores emerged in clusters, each bearing a denticulate rachis at the apex. These conidiophores measured between 2.7-4.8 μm in width. As the fungus advanced to the sporulation stage, the majority of conidia were produced in clusters, taking on a globose to sub-globose shape (Plate 1e). Conidia.
These measured between 1.9-2.6 μm in length and 1.8-2.5 μm conidiophores had a sub-spherical to ampulliform base, in width.
Table 1. Measurements of morphological structures of different B. bassiana isolates identified from survey

Table 2. Measurements of morphological structures of different M. anisopliae isolates identified from survey

Isolate 6. B. bassiana. Utukur, Kadapa (Bb. Utrr. Kdp )
The white cottony growth was observed on SDAY medium with simultaneous sporulation of mycelium. Mycelia measured to be between 1.1-2.1 μm in width. Conidiophores are solitary but usually in clusters of four or more, base sub-spherical to ampulliform and 3.0-4.9 µm wide, apex with denticulate rachis. Conidia are produced in clusters globose or subglobose in shape (Plate 1). Conidia were measured from 1.9-2.7 μm in length and 1.6-2.4 μm in width.
In the present study of B. bassiana isolates, the growth of mycelia was puffy with abundant sporulation on SDAY medium. The white powdery sporulation was observed in the centre part, surrounded by whitish mycelium. Conidia were raised from conidiophores. Conidiophores are solitary but usually in clusters of two or more. The majority of the conidia was oval to globose and sub globose in shape (Plate 1). Mycelia width was measured and ranged between 1.0- 2.2 μm in width. Conidiophores ranged in width from 2.7 – 5.2 μm. Conidia were measured from 1.9 – 3.1 μm in length and 1.6 – 2.8 μm in width (Table 1f).
The isolates of B. bassiana Bb. Dpl. Ctr, Bb. Pmr. Ctr, Bb. Pkd. Knl, Bb. Dvk. Knl, Bb. Gty. Atp, and Bb. Utr. Kdp were found to be more or less identical in their morphological characteristics.
Morphological structures of M. anisopliae isolates
After purifying the cultures, the morphology of six M. anisopliae isolates was studied. Two days after inoculation of M. anisopliae isolates on SMAY medium, the beginning of mycelial development was seen as white to yellowish small portion. The mycelium was creamy white or yellowish in colour. During sporulation, the colonies became greenish in colour. After 10- 15 days, it was changed to dark green in colour
Microscopic examination of mycelia threads in isolates indicated that they were septate mycelia and interwoven. Mycelium (hypha) width ranged from 3.0 to 4.5 μm.
Isolate 7. Metarhizium anisopliae, Dandapalle, Chittoor (Ma. Dpl. Ctr )
apOpenarSaMncAe,Ywmitehdisam, ocotlhonmieyscweleiareasnodmseiwmhualtarnaeioseuds sporulation is observed. The colony of this isolate presented a white edge with sporulation observed mostly in the centre part. Mycelia was measured to be between 3.3 to 4.1 μm in width. Conidiophores were less and not branched. Conidiophores ranged in width from 4.0 to 6.3 μm. Conidia were produced directly from conidiophores and the majority of the conidia were elongated with broader ends (Oblong) and cylindrical in shape (Plate 2a). Conidia were measured from 6.5 to 7.8 μm in length and 3.5 to 4.0 μm in width.
Isolate 8. Metarhizium anisopliae, Palamaner, Chittoor (Ma. Pmr. Ctr )
The growth of mycelia on SMAY media was observed as a fine, white to pale yellowish mycelial mat. This early stage typically forms a cottony or velvety texture, with the fungus spreading radially across the medium with simultaneous abundant sporulation. The dark green sporulation was seen in the centre part, surrounded by pale yellowish mycelium. Mycelia width
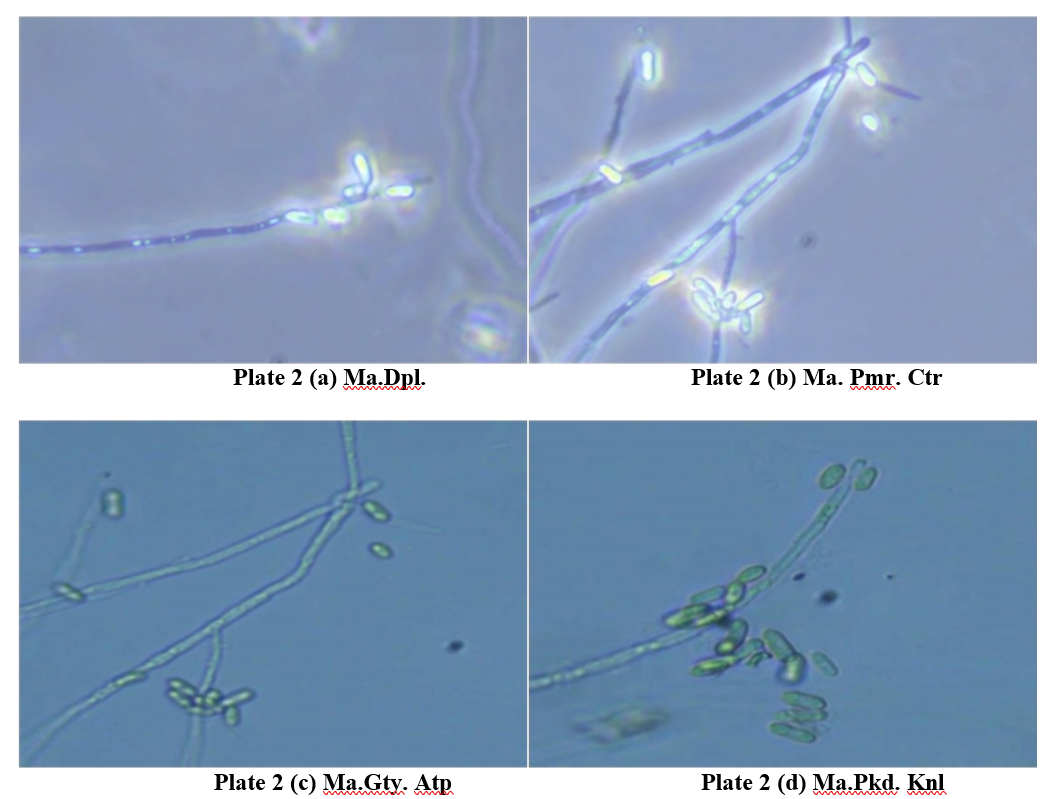
was measured to be between 3.0 and 3.6 μm in width. branched conidiophores. Conidiophores ranged in width No branched conidiophores. Conidiophores ranged in from 4.3 to 6.1 μm. The majority of the conidia were width from 4.3 to 6.0 μm. Conidia were raised from elliptical and elongated in shape (Plate 2e). Conidia were the conidiophores. The majority of the conidia were measured from 6.4 to 7.5 μm in length and 3.4 to 3.6 μm elliptical and elongated in shape (Plate 2b). Conidia were in width. measured from 6.7 to 7.5 μm in length and 3.2 to 3.5 μm in width.
Isolate 9. Metarhizium anisopliae, Pattikonda, Kurnool (Ma. Pkd. Knl)
The growth of this isolate on SMAY medium began with the development of white to yellowish mycelia, followed by the simultaneous sporulation after 7 days. Mycelial strands measured between 3.2 to 3.7 μm in width, while conidiophores ranged from 4.0 to 5.9 μm in width. Conidia were produced directly from the conidiophores, which were observed to be unbranched in this instance. The conidia displayed an elongated ellipsoidal to cylindrical shape (Plate 2c). The conidia were measured to be 6.0 to 7.1 μm in length and 3.1 to 3.3 μm in width.
Isolate 10. Metarhizium anisopliae, Devanakonda, Kurnool (Ma. Dvk. Knl )
On SMAY medium, the growth of fungi began with the appearance of white to yellowish hyphae, spreading radially across the plate. The colony exhibited a distinct white edge, while sporulation was primarily concentrated in the central region. The conidiophores were sparse and unbranched, producing conidia directly. The width of the mycelia was measured between 3.2 and 4.1 μm, while the conidiophores ranged from 4.2 to 6.0 μm in width. The majority of the conidia displayed an elongated shape, sporulation stages.
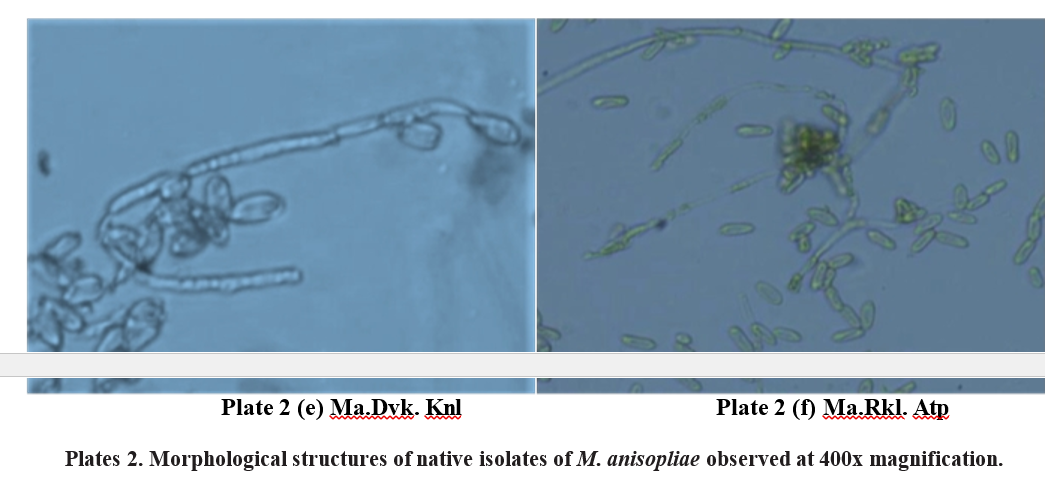
Isolate11 .Metarhiziumanisopliae ,Gooty,Anantapuramu (Ma. Gty. Atp )
The growth of mycelia was raised form with simultaneous abundant sporulation of M. anisopliae on SMAY medium in the petriplate was observed. The dark green sporulation was seen in the centre part, surrounded by pale yellowish mycelium. Mycelia width was measured to be between 3.0 and 3.6 μm in width. Conidia were raised from the conidiophores. No branched conidiophores. Conidiophores ranged in width from 4.3 to 6.1 μm. The majority of the conidia were elliptical and elongated in shape (Plate 2e). Conidia were measured from 6.4 to 7.5 μm in length and 3.4 to 3.6 μm were in width.
Isolate 12. Metarhizium anisopliae, Rekulakunta, Anantapuramu (Ma. Rkl. Atp )
The growth of mycelia was observed as white to yellowish mycelia with simultaneous abundant sporulation of M. anisopliae on SMAY medium in the petriplate was observed. Conidia were raised from the conidiophores. No branched conidiophores. The majority of the conidia were elongated ellipsoidal to cylindrical in shape (Plate 2f). Mycelia width was measured to be between 3.2 to 3.7 μm. Width of the conidiophores ranged from 4.0 to 6.2 μm. Conidia were measured from 6.7 to 7.2 μm in length and 3.2 to 3.5 μm in width.
In the present study of M. anisopliae isolates, the growth of mycelia was raised form with concurrent abundant sporulation of M. anisopliae on SMAY medium was seen. The dark green sporulation was observed in the centre part, surrounded by yellowish to white mycelium.
Conidia were raised from conidiophores. Conidiophores were less branched or had no branches. The majority of the conidia were elongated with broader ends (Oblong) and round in shape. The mycelial width of M. anisopliae isolates ranged between 3.0 to 4.1 μm, conidiophore width ranged between 4.0 to 6.3 μm. Conidia were measured from 6.0 to 7.8 μm in length and 3.1 to 4.0 μm in width. (Table 2)
The results of Ma. Dpl. Ctr, Ma. Pmr. Ctr, Ma. Pkd. with broader elliptical ends or cylindrical forms (Plate Knl, Ma. Dvk. Knl, Ma. Gty. Atp, Ma. Rkl. Atp isolates 2d). The conidia measured between 6.3 and 7.2 μm in measurements revealed that different structures of M. length and 3.2 to 4.0 μm in width. This sequential growth anisopliae had more or less identical measures. The pattern reflects typical morphological development, mycelial width of M. anisopliae isolates ranged between with a clear differentiation between hyphal growth and 3.0 to 4.5 μm, conidiophore width ranged between 4.0 sporulation stages. to 7.4 μm. Conidia were measured from 6.0 to 7.9 μm in length and 3.1 to 4.0 μm in width.
The results are in conformity with Neto et al. 2024) whoreported that B. bassiana presents white to yellowish white colonies with a woolly to cottony appearance on media. Colonies are composed of aerial hyphae and aggregates of conidiogenic hyphae and conidia, which form spherical clusters (conidiophores). Microscopically, the fungus has hyaline, septate and branched hyphae with smooth walls, measuring up to 2.5 μm thick. Conidiogenic hyphae are either in groups to powdery mycelia with globose conidia, while M. or single and have an ampulliform basis. Conidia (1.9– anisoplia edevelope ddar kgree ncolonie swit hcylindrical 2.7×1.4–1.8 μm) are hyaline, globose to subglobose, and conidia. In B. bassiana Conidia were measured from 1.9 not septate and have thin, smooth walls.
The presesnt findings are also in agreement with the findings of Cokola et al. (2023) who reported that conidia from B. bassiana isolate P5E were slightly larger than those from KA14 in size, on average. Conidial measurements were variable and ranged from 2.4-3.6 µm in length and from 1.8 to 3.0 µm in width. The mean conidial length was 3.2±0.32 µm for isolate P5E versus 2.7±0.21 µm for isolate KA14. The largest value of conidial width was recorded in isolate P5E (2.45±0.27 µm) compared to isolate KA14 (2.34±0.17 µm).
The results are in line with Gowri (2022) who stated in the morphological study of four isolates of M. anisopliae, conidiophores were found to be few branched to no branches, Aseptate, and wider than mycelium in the majority of isolates. The shape of the conidia was elongated with broader ends, round and elliptical conidia. Mostly, similar measurements were found in all the isolates. The mycelial width of M. anisopliae isolates ranged between 3.3 to 4.5 μm, conidiophore width ranged between 4.3 to 7.7 μm. Conidia were measured from 6.0 to 8.0 μm in length and 3.2 to 4.0 μm in width. The colonies grow from white to dark green to brownish green in colour. Similarly, Thaochan and Sausaard (2017) categorized M. anisopliae conidial morphologies according to length to width (L/W) ratio into four groups: 1.69-1.96, 2.01-2.47, 2.55-2.94 and 3.14-3.16.
Bai et al. (2015) observed that colony borders of all the isolates of M. anisopliae were regular. During sporulation, the colour of colonies became yellow green or olivaceous green to dark green. The average spore width was 2.10- 4.10 μm, while the average spore length was 3.20- 7.69 μm. The spores were categorized as oval, round, or elongated based on their length and width. Sepulveda et al. (2016) studied the six promising strains of Metarhizium spp. The average conidia length and width for the six strains were 5.09 μm and 1.92 μm, respectively.
The morphological characterization of native Beauveria bassiana and Metarhizium anisopliae isolates shows distinct growth and sporulation patterns, providing essential markers for initial identification in biocontrol efforts. B. bassiana isolates consistently displayed white in case of M. anisopliae, Conidia were measured from 6.7 to 7.2 μm in length and 3.2 to 3.5 μm in width. Future studies should integrate molecular techniques to complement morphological data, enhancing species identification and insights into genetic diversity. This approach is vital for optimizing entomopathogenic fungi use in pest management, supporting precision and sustainability in agriculture.
LITERATURE CITED
Bai, N.S., Sasidharan, T.O., Remadevi, O.K., Dharmarajan, P., Pandian, S.K and Balaji, K. 2015. Morphology and RAPD analysis of certain potentially entomopathogeni cisolate sofMetarhiziumanisopliae Metsch. (Deuteromycotina: Hypocreales). Journal of Microbiology and Biotechnology Research. 5(1): 34- 40.
Boucias, D., Stokes, C., Suazo, A and Funderburk, 2000. AFLP analysis of the entomopathogen Nomuraea rileyi. Mycologia. 92: 638-648.
Cokola, M.C.,Fekih, B.I., Bisimwa, E.B., Megido,C. R., Delvigne, F and Francis, F. 2023. Natural occurrence of Beauveria bassiana (Ascomycota: Hypocreales) infecting Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) and earwig in eastern DR Congo. Egyptian Journal of Biological Pest Control. 33(1): 54.
Fernandes, E.K.K., Keyser, C.A., Chong, J.P., Rangel, D.E.N., Miller, M.P and Roberts, D.W. 2009. Characterization of Metarhizium species and varieties based on molecular analysis, heat tolerance and cold activity. Journal of Applied Microbiology. 108: 115-128.
Gowri, V. 2022. Morphologicalandmolecularcharacterization of Metarhizium rileyi and Metarhizium anisopliae. M.Sc.(Ag.)Thesis .AcharyaN.G.RangaAgricultural University Lam, Guntur.
Manjula, K., Saheb, P.Y., Sudheer, J.M and Rao, R.A. 2019. First Record on Occurrence of Epizootics of Metarhizium anisopliae (Metchnikoff) Sorokin on Groundnut Leaf Miner, Aproaremamodicella (Deventer). International Journal of Current Research. 11(4): 2715-2717.
Neto, S.C.D.M., Calaça, F.J.S., de Souza, W.G., Santos, L.A.C., de Pina, I.J., Xavier-Santos, S. and Calil, F.N., 2024. First natural occurrence of the entomopathogenic fungus Beauveria bassiana (Bals. Criv.) Vuill. on Cosmopolites sordidus (Germar, 1824)(Curculionidae, Coleoptera) in an agroforestry system in the Brazilian Cerrado. Check List. 20(1): 47
Nishi, O., Iiyama, K., Yasunaga-Aoki, C and Shimizu, 2011. Incongruence between EF1α Phylogeny and Morphology of Metarhizium majus and Metarhizium guizhouense in Japan. Entomotech. 34:19-23.
Sepulveda, M., Vargas, M., Gerding, M., Ceballos, R and Oyarzua, P. 2016. Molecular, morphological and pathogenic characterization of six strains of Metarhiziumspp .(Deuteromycotina:Hyphomycetes) forthecontrolofAegorhinussuperciliosus(Coleoptera: Curculionidae). Chelian. Journal of Agricultural Research. 76(1): 77- 83.
Thaochan, N and Sausard, W. 2017. Occurrence and effectiveness of indigenous Metarhizium anisopliae against adults Zeugodacuscucurbitae (Coquillett) (Diptera: Tephritidae) in Southern Thailand. Songklanakarin. Journal of Science and Technology. 39(3): 325-334.
Toledo, A.V., De Remes Lenicov, A.M.M and Lastra L.Y.C.C. 2008. Host range findings on Beauveria
bassiana and Metarrhizium anisopliae (Ascomycota: Hypocreales) in Argentina. Bulletin of the Botanical Society Argentina. 43(3-4): 211-220.
- Effect of Sowing Window on Nodulation, Yield and Post – Harvest Soil Nutrient Status Under Varied Crop Geometries in Short Duration Pigeonpea (Cajanus Cajan L.)
- Nanotechnology and Its Role in Seed Technology
- Challenges Faced by Agri Startups in Andhra Pradesh
- Constraints of Chcs as Perceived by Farmers in Kurnool District of Andhra Pradesh
- Growth, Yield Attributes and Yield of Fingermillet (Eleusine Coracana L. Gaertn.) as Influenced by Different Levels of Fertilizers and Liquid Biofertilizers
- Consumers’ Buying Behaviour Towards Organic Foods in Retail Outlets of Ananthapuramu City, Andhra Pradesh

