Integrated Disease Management (Idm) Of Tobacco Streak Virus In Groundnut
0 Views
M. SUNIL KUMAR*, R. SARADA JAYALAKSHMI DEVI, K. VEMANA, T. MURALI KRISHNA AND L. PRASANTHI
Department of Plant Pathology, S.V. Agricultural College, ANGRAU, Tirupati.
Tobacco streak virus (TSV) is the type member of Ilarvirus genus in the family Bromoviridae (Fauquet et al., 2005). The genus Ilarvirus is one of the six genera included in the family Bromoviridae and is the largest genus in the family with more than 15 members and seven or eight subgroups (Hull, 2002). Tobacco streak virus, being a destructive virus of economically important crops, knowledge on disease diagnosis, reliable detection method and characterization plays key role in disease management.
As the TSV transmission is assisted by thrips vector, present emphasis has been given more on management of thrips by using border cropping with sorghum and different insecticides. Continuous use of chemicals led to residual toxicity and health hazards, besides increasing cost of cultivation due to higher input costs. The perusal of literature pertaining to the management of the TSV indicates that very few efforts were made to integrate or combine the available compatible methods of control for efficient management of TSV in groundnut. So, in order to evolve an effective integrated approach for management of TSV in groundnut, the present investigation was undertaken with the integration of different modules or treatments using susceptible groundnut cv. K-6 under field conditions for two consecutive years during Kharif seasons (2014-15 and 2015-16) in Randomized Block Design (RBD) at Agricultural Research Station (ARS), Kadiri, Andhra Pradesh.
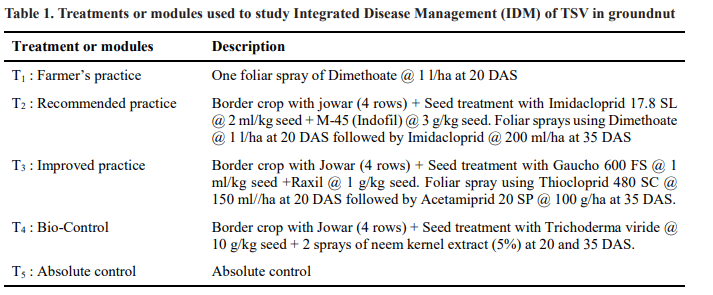
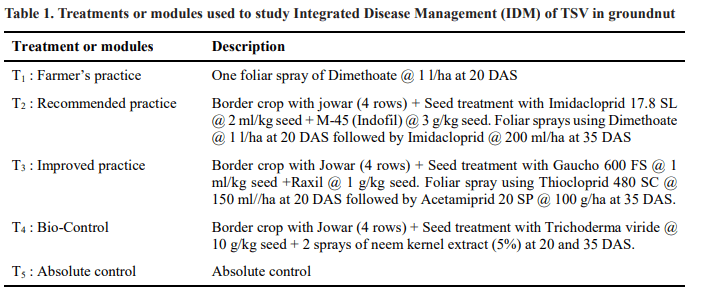
Sowings were done on the first day of second fortnight of june month during two consecutive kharif seasons. The details of the treatments or modules are given in Table 1.
The net plot size of 5m X 4m was maintained for each treatment with 60 cm distance between rows and 30 cm distance between plants. Seed treatment with imidacloprid 600FS @ 2 ml/Kg was done prior to sowing and all the recommended package of practices was followed and the plots were irrigated whenever necessary. Weeding was done manually twice at 15 and 30 Days After Sowing (DAS). Seeds of sorghum were sown in three rows 15 days prior to groundnut sowing as a border crop with a spacing of 45 X 30 cm around the treatment plots.
In treatments where rouging is one of the components, the TSV infected plants were removed and destroyed as and when observed after recording incidence of TSV. Subsequently next rouging was taken up after 15 days.
The insecticide application was done as per the schedule and dosages mentioned in each treatment, wherein first foliar application of the insecticides was done at 15 DAS followed by the other applications at 30 and 45 DAS using a hand compression sprayer during evening hours. Care was taken to ensure complete drenching of the plants in each treatment and drift avoided.
In each treatment, data on population of thrips incidence of the disease were recorded. The data collected in different observations were statistically analysed and pooled analysis of both the seasons provided as per the design.
The results revealed significant differences among the treatments pertaining to PSND incidence at 7 and 14 days after 1st and 2nd spraying. All treatments were significantly different when compared to control with reference to PSND incidence at 7 and 14 days after 1st
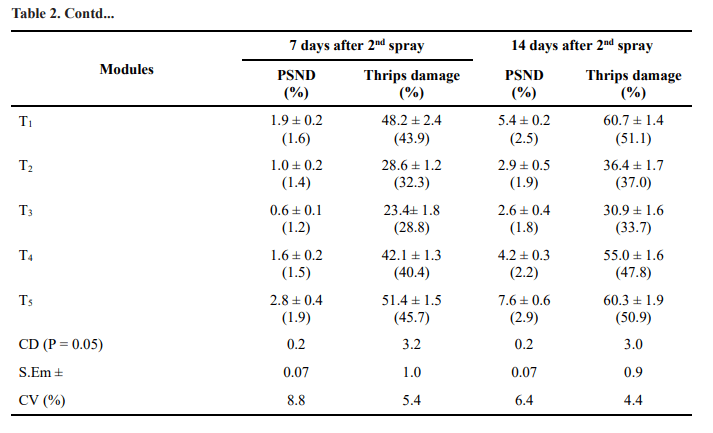
spraying. Lowest (0.2 and 0.3 per cent) PSND incidence was recorded in Treatment 3 (T3) followed by Treatment 2 (T2) (0.4 and 0.7 per cent) (Table 2).
Significant differences were observed among the different modules or treatments with respect to thrips damage at 7 and 14 days after 1st and 2nd spraying. Pooled data for both years Kharif 2014-15 and Kharif 2015-16 revealed that Treatment 3 (T3) recorded lowest thrips damage per cent of 14.6 and 21.2 at 7 and 14 days after 1nd spraying which was significantly different from other treatments and control. The next best treatment is recommended practice (T2) with per cent thrips damage of 19.3 and 27.2 per cent at 7 and 14 days after 1st spraying which is significantly different when compared to control (T5) (Table 2).
Similar attempts were made to develop management strategies against TSV in groundnut and other crops by using various Treatments or modules. Halakeri (2006) reported that the spread of Tobacco streak virus could be minimized by spraying Imidacloprid (0.025%). Use of border crops like sorghum reduced the incidence of disease from 18 to 7 per cent. Similarly spray of imidacloprid @ 0.05 per cent at 30 DAS reduced the incidence of disease from 27 to 5 per cent (Mesta et al., 2003). Seed treatment with imidacloprid (5g/kg) + spray (@ 0.25ml/L) at 30, 45 and 60 days after sowing and sorghum as a border crop, by way of reducing the vector movement kept the TSV incidence at low (Halakeri, 2006). Similarly, Mesta et al. (2003) reported that use of sorghum border crop reduced the incidence of TSV in sunflower from 18 to 7 per cent and seed treatment and spray of imidacloprid @ 0.05 per cent at 30 DAS reduced disease incidence from 27 to 5 per cent. Besta (2004) reported that, the seed treatment (5 g/kg) with imidacloprid followed by confidor spray (0.5 ml/L) inhibited the disease and increased yield (90.52% and 13.61 q/ha, respectively) as compared to control (40.12 PDI and 5.78 q/ha yield). Shirshikar (2008) revealed that if the sunflower crop is bordered with Sorghum and treated with imidacloprid (Gaucho 70 W.S., 5 g / kg) along with spraying of imidacloprid (Confidor 200 S.L. 005 %) three sprays at 15, 30 and 45 days after sowing, the incidence of sunflower necrosis diseases can be minimized. Shirshikar (2010), reported that sunflower necrosis disease can be managed by treating seeds with Thiamethoxam at 4 g/kg along with two sprays of the chemical at per cent 30 and 45 days after sowing.
No single method can currently provide adequate control of vector transmitted virus diseases. However, integrated management systems using moderately resistant cultivars and suppressive chemicals and cultural practices have been developed and successfully deployed for minimizing losses caused by viruses in various crop plants. Although the impact of management practices developed in the present study is encouraging, the disease continues to pose threat to groundnut production. Concerned efforts in research for identification of resistant sources in germplasm and further incorporation and development of varieties/hybrids must be continued to sustain progress in understanding the factors that contribute to epidemics of the disease and in developing improved strategies for disease management.
LITERATURE CITED
Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U and Ball, L.A. 2005. Virus taxonomy. VIIIth Report of ICTV, Academic press, New York, USA.1259.
Hull, R. 2002. Genus Ilarvirus. In: R. Hull (ed.) Matthew’s Plant Virology, 4th edn. Academic Press, San Diego.
Halakeri, V. 2006. Studies on serodiagnosis, epidemiology and management of Sunflower necrosis viral disease in northern Karnataka. M.Sc. (Ag.) Thesis, University of Agricultural Sciences, Dharwad.
Mesta, R.K., Katti, P and Hosamani, A. 2003. Management of sunflower necrosis disease by vector control. Proceeding of Recent Development in the Diagnosis and Management of Plant Diseases for Meeting Global Challenges, University of Agricultural Sciences, Dharwad, 18-20 December, 2020. Indian Phytopathological Society, New Delhi. 37.
Besta, G. 2004. Epidemiology and management of sunflower necrosis disease. M.Sc. (Ag.) Thesis. University of Agricultural Sciences, Dharwad.
Shirshikar, S.P. 2008. Sunflower necrosis disease management with thiomethoxan. Helia. 33 (53): 63- 68.
Shirshikar, S.P. 2010. Control of sunflower necrosis disease with new chemicals. Journal of Oil Seed Research. 26(S): 484-486.
- Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
- Brand Preference of Farmers for Maize Seed
- Issues That Consumer Experience Towards Online Food Delivery (Ofd) Services in Tirupati City
- Influence of High Density Planting on Yield Parameters of Super Early and Mid Early Varieties of Redgram (Cajanus Cajan (L.) Millsp.)
- Influence of Iron, Zinc and Supplemental N P K on Yield and Yield Attributes of Dry Direct Sown Rice
- Effect of Soil and Foliar Application of Nutrients on the Performance of Bold Seeded Groundnut (Arachis Hypogaea L.)

