Influence of Nano Chitosan Encapsulated Growth Hormones on Morpho-Physiological and Yield Attributes of Groundnut
0 Views
S. T. MONISHA*, A. R. NIRMAL KUMAR, P. LATHA AND T. N. V. K. V. PRASAD
Department of Crop Physiology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
Groundnut (Arachis hypogaea L.) is a vital leguminous oilseed crop rich in oil and protein, extensively cultivated in tropical and subtropical regions. In India, it occupies 48.80 lakh hectares with a productivity of 1,847.4 kg/ha. Plant growth regulators (PGRs) such as GA3, auxins and cytokinins enhances growth, nodulation, and yield. The treatment with GA3 at 50 ppm significantly improved pod and seed development. Nanoparticles, particularly nano-chitosan, facilitate targeted and sustained delivery of PGRs, though research in groundnut remains limited. An experiment titled “Influence of nano chitosan encapsulated growth hormones on morpho-physiological attributes of groundnut” was conducted during kharif 2024 at wetland farm, S.V. Agricultural College, Tirupati. Treatments included nano-encapsulated GA3, auxins, and cytokinins, with observations on growth and yield parameters. Significant variability was observed among eight treatments in groundnut under the influence of nano chitosan encapsulated growth promoters. At 60 DAS, maximum plant height was recorded in T2 (GA3 @ 50 ppm) 31.11 cm. The highest number of branches (5.67) and stem diameter (1.78 mm) were noted in T5 (Nano chitosan encapsulated GA3 @ 50 ppm). While differences in SPAD chlorophyll meter readings and Leaf area index were not statistically significant, T5 consistently exhibited superior values. These results highlighted the potential of nano chitosan-based GA3 for enhancing groundnut vegetative growth. Nano chitosan encapsulated GA3 recorded the highest seed yield (2533.2 kg ha-1), harvest index (41.36 %) and haulm yield (45.89 g). Results showed that the improved plant responses under nano-chitosan encapsulated PGR treatments, indicating their potential for enhancing groundnut productivity.
KEYWORDS: Nano chitosan, NAA, GA3, BAP.
INTRODUCTION
Groundnut (Arachis hypogaea L.) is derived from two Greek words, Arachis meaning a legume and hypogaea meaning below ground, referring to the formation of pods in the soil. Groundnut is an upright annual plant. It is an important leguminous oilseed crop. Generally distributed in the tropical, sub-tropical and warm temperate zones. It contains high oil content and edible seeds. It is the fourth most important source of oil and third most important source of vegetable protein in the world.
Groundnut is an economically important oilseed crop cultivated in an area of approximately 373 lakh hectares globally, with production of 559 lakh tonnes and productivity of 1,656 kilograms per hectare (FAOSTAT, 2024). In India groundnut covers an area about 48.80 lakh hectares and production was estimated about 102.89 lakh tonnes with a productivity of 1847.4 kg/ha. In Andhra Pradesh groundnut covers area about 3.66 lakh ha and production is estimated about 3.56 lakh tonnes with an average productivity of 1141 kg ha-1 (Crop Outlook Reports of Andhra Pradesh, 2023-24).
Auxins play an important role in gravitropic response and Amyloplast accumulation and Spatial distribution during the peg development in groundnut. Cytokinin at the tissue and organ levels include the differentiation of phloem and meta xylem in roots regulation of cell division, photomorphogenic cell differentiation in expanding leaves and shoots inhibition of leaf senescence.
Nanoparticles act as a bridge in between atomic/ molecular structures and their bulk counterparts, and thus are known to have great scientific interest. The use of nano-carriers for the application of plant growth regulators (PGRs) can ensure the slow delivery and sustained release of bioactive components, thereby avoiding their supra-optimal levels. The use of nanoparticles in the modern system of agriculture is highly advantageous due to the effective delivery of agrochemicals at the targeted location, mainly because of larger surface area, high mass transfer rate and easy attachment of applied chemicals.
Chitosan is derived from arthropods exoskeleton and fungi cell walls and the second renewable carbon source after lignocellulosic biomass and effective nutrient delivery material. Further, the study on effect of nano chitosan encapsulated plant growth promoters on morphological, physiological and biochemical aspects of crops is meagre.
MATERIAL AND METHODS
The experiment entitled “Influence of nano chitosan encapsulated growth hormones on morpho-physiological attributes of groundnut (Arachis hypogea L.)” was conducted during kharif, 2024 in wetland farm, S.V. Agricultural College, Tirupati campus of Acharya N.G. Ranga Agricultural University which is geographically at 79°E longitude and 13°N latitude was used for each treatment.
Treatments:
T1 – Auxin (NAA) @ 50 ppm
T2 – GA3 @ 50 ppm
T3 – Cytokinin (BAP) @ 50 ppm
T4 – Nano chitosan encapsulated NAA @ 50 ppm T5 – Nano chitosan encapsulated GA3 @ 50 ppm T6 – Nano chitosan encapsulated BAP @ 50 ppm T7 – Nano chitosan @1 %
T8 – Normal chitosan
Note
NAA- Naphthalene acetic acid GA- Gibberellic acid
BAP- Benzyl amino purine
Stages of Spray: 30 DAS and 60 DAS Stages of observation: 30, 60 and 90 DAS
1. Morpho-physiological attributes
- Plant Height (cm)
Three plants were collected randomly and measured the p lant height from base of the plant to shoot tip and expressed in centimeters (cm).
1.2 Number of Branches (no.)
Three plants were collected randomly from each plot and number of branches per plant were counted.
1.3 Stem Diameter (mm)
Three plants were collected randomly and measured the stem diameter by using digital vernier calliper and average value were recorded.
1.4 SCMR (SPAD chlorophyll meter reading)
SCMR was measured by using of SPAD (SPAD- 502, Minolta corp., Ramsey, NJ). Three plants were selected randomly and taken the reading of every third leaf from the top of each plant in each plot, averaged.
1.5 Leaf Area Index (LAI)
The total leaf area was measured using a Leaf Area Meter (LICOR, Model LI 3000) and the results were expressed as cm2 plant-1. The leaf area index was calculated as follows by employing the formula of Williams (1946).

2. Yield attributes
2.1 Haulm Yield Plant-1
The total dry matter was estimated from the three randomly selected plants sampled from each treatment in three replications. The plants were kept in hot air oven for drying at 80oC for two days and the dry weights were recorded and expressed in gm plant-1.
2.2 Seed Yield kg ha-1
After harvesting the plants were shade dried for
5 days. After complete drying of the plants, weight of kernels of an individual plants were collected and recorded in gm plant-1.
2.3 Harvest Index
Harvest index (HI) is defined as the ratio of economic yield to total biological yield (Donald and Humblin, 1976) and expressed in percentage. Harvest index was calculated by using the formula,
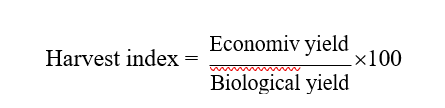
3. RESULTS AND DISCUSSION
Influence of nano chitosan encapsulated growth promoters on groundnut revealed significant variability on morpho-physiological and yield parameters. The data provided in the Table 1. represents the comparative performance of eight different treatments in groundnut.
3.1 Plant Height (cm)
Plant height is an important character of the vegetative phase and indirectly influences the yield components. In this study, maximum plant height was recorded in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 27.41 cm and minimum plant height was observed in T8 (control) 22.28 cm at 30 DAS. No significant difference was observed in plant height at 60 DAS. In T5 (Nano chitosan encapsulated GA3 @ 50 ppm) highest plant height (38.00 cm) was recorded at 90 DAS. When gibberellic acid is applied as a foliar spray, it leads to an elongation of the hypocotyl and the two nodes directly above it, which in turn contributes to an overall increase in plant height. Patil (2019) also reported comparable results, indicating that the application of GA3 lead to a significant increase in plant height in groundnut. This outcome is consistent with the findings reported by Emongor (2007).
3.2 Number of Branches (no.)
Branches are the site of the leaves, flower and peg formation. So, number of branches is desirable attribute for higher biomass production and yield. Number of branches per plant was observed maximum in T2 (GA3 @ 50 ppm) 4.44 which is on par with T6 (Nano chitosan encapsulated BAP @ 50 ppm) 4.00 at 30 DAS. No significant difference was observed at 60 and 90 DAS. Highest number of branches observed in T6 (Nano chitosan encapsulated BAP @ 50 ppm) 6.67 and lowest branches recorded in T4 (Nano chitosan encapsulated NAA @ 50 ppm) at 90 DAS. Hong Yan and Shu Yu (2001) stated that number of branches might be due to increased number of internodes or length of internodes because of increased cell number in soybean.
3.3 Stem Diameter (mm)
Groundnut stem supports leaves, flowers, and pegs for pod development. A continuous increase in stem diameter was observed from 30 DAS to 45 DAS irrespective of treatments. Significance difference was recorded among the treatments at 60 DAS. Maximum stem diameter was observed in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 1.41 mm and minimum diameter was found in T1 (NAA @ 50 ppm) and T8 (control) 1.00 mm at 30 DAS. At 60 DAS, T3 (BAP @ 50 ppm) recorded the highest stem diameter (2.36 mm) and T2 (GA3 @ 50 ppm) recorded minimum stem diameter (1.51 mm) which is on par with T1 (NAA @ 50 ppm)1.53mm. No significance difference was observed at 90 DAS where T3 (BAP @ 50 ppm) showed maximum stem diameter (3.27 mm) and minimum in T2 (GA3 @ 50 ppm) 2.10 mm. Leite et al. (2003) also reported the increase stem diameter in soybean with foliar application of cytokinin. BAP (6-benzylaminopurine) increases stem diameter in groundnut mainly because it promotes cell division and expansion, especially in the vascular and cambial tissues of the stem. Ozkurt and Bektas (2022) reported that chitosan application improved various growth parameters, including an increase in shoot diameter in tomato plants.
3.4 SPAD Chlorophyll Meter Reading (SCMR)
Chlorophyll is a photosynthetic pigment, plays a pivotal role in capturing sunlight and then converting it to luminous energy. Irrespective of treatments imposed SCMR values increased from 30 DAS to 75 DAS. No significant difference was observed among treatments. Maximum SCMR value recorded in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 38.1 and lowest was observed in T2 (GA3 @ 50 ppm) 35.7 followed by T7 (Nano chitosan @ 1 %) 35.8 at 30 DAS. At 60 DAS, T2 (GA3 @ 50 ppm) recorded the highest SCMR readings (42.9) followed by T6 (Nano chitosan encapsulated BAP @ 50 ppm) 42.5 and T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 42.1. At 90 DAS, SCMR values decreased and lowest was recorded in T7 (Nano chitosan @ 1 %) 38.8.
Saini et al. (2016) stated that maximum total chlorophyll content was observed in NAA and GA3 treatments. NAA and other plant growth regulators improve chlorophyll content by boosting nitrogen efficiency, encouraging chloroplast development, and
Table 1. Influence of nano chitosan encapsulated growth hormones on morpho-physiological attributes of groundnut
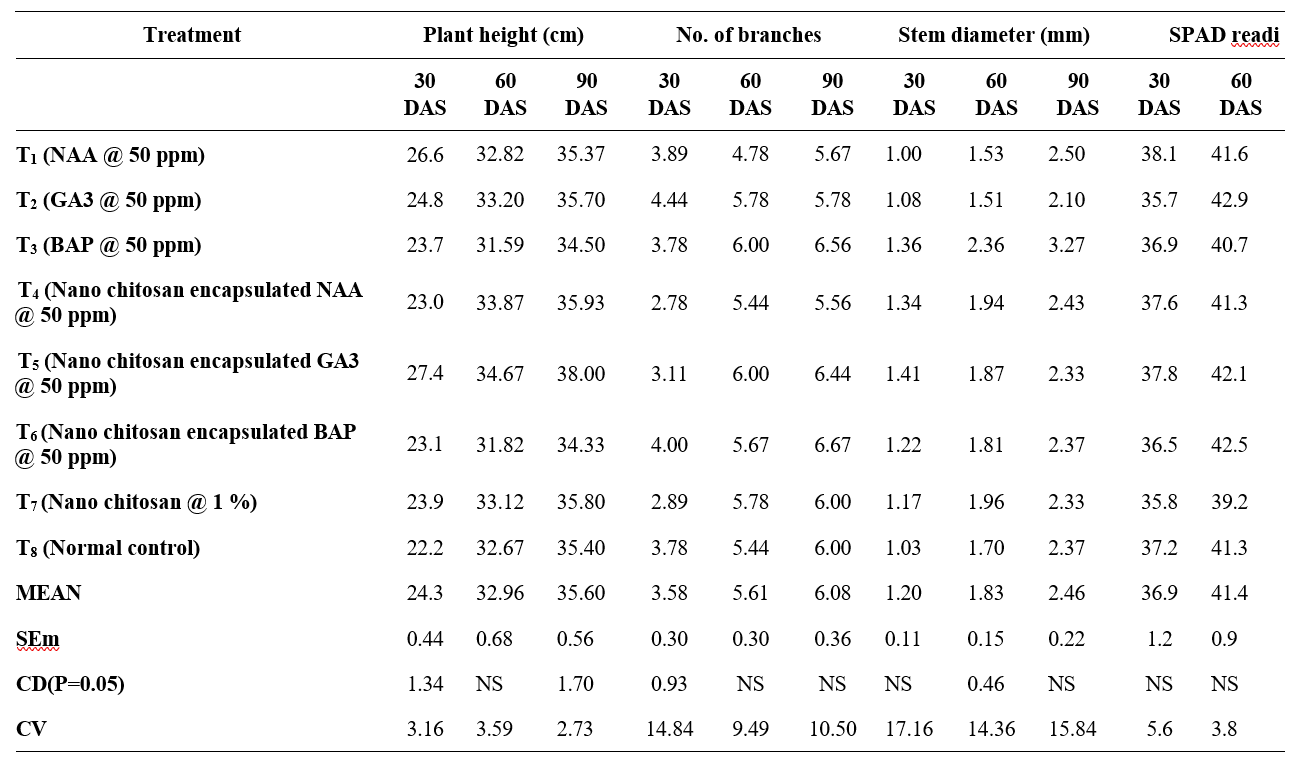
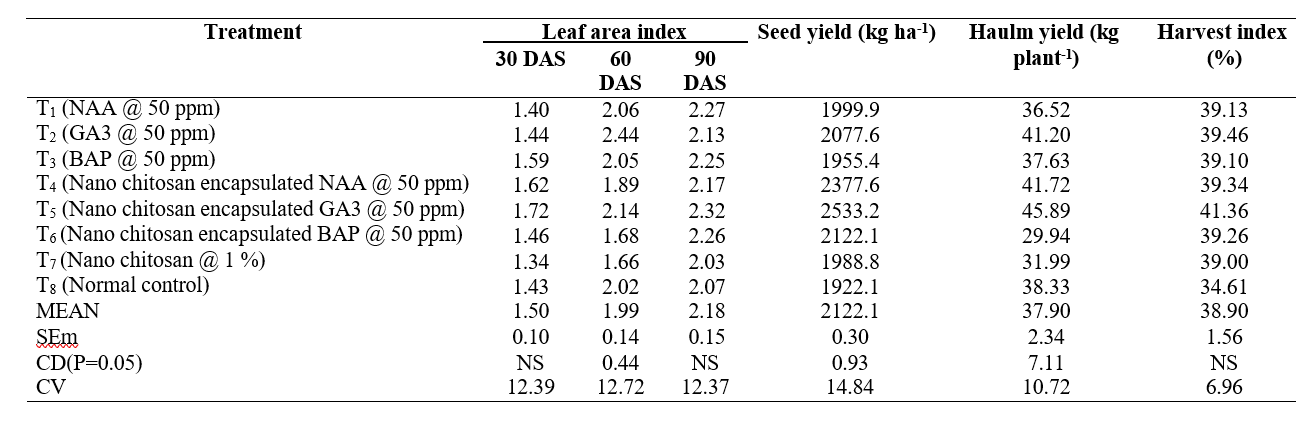
Table 2. Influence of nano chitosan encapsulated growth hormones on morpho-physiological and yield attributes of groundnut
delaying leaf aging. These effects help plants produce, retain, and increase chlorophyll levels more effectively.
3.5 Leaf Area Index (LAI)
Leaf area index is determined by the ratio between leaf area and space occupied by the crop and it is one of the main factors influence the photosynthetic rate of canopy. The results indicated that the LAI was continuously increased from 30 DAS to 90 DAS. No significant difference was observed among the treatments. However, at 30 DAS, T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 1.72 recorded the highest LAI and lowest was seen in T7 (Nano chitosan @ 1 %) 1.34. At 60 DAS, minimum LAI was recorded in T7 (Nano chitosan @ 1 %) 1.66 followed by T6 (Nano chitosan encapsulated BAP @ 50 ppm) 1.68 and maximum observed in T2 (GA3 @ 50 ppm) 2.44. At 90 DAS, lowest LAI recorded in T7 (Nano chitosan @ 1 %) 2.07 and highest in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 2.32. Surendar et al. (2013) reported that foliar application of plant growth regulators in blackgram significantly increased the Leaf area index, Crop growth rate, Net assimilation rate and Specific leaf weight by showing higher accumulation of total dry matter production with increased yield.
3.6 Seed Yield kg ha-1
Source-sink relation contributes the seed yield. It includes phloem loading at source (leaf) and unloading at sink (seed and pod) by which the economic part will be getting assimilates synthesized by photosynthesis, resulted by Gardner et al., (1988). No significant difference was observed in seed yield in groundnut among all treatments. Highest seed yield was observed in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 2533.2 kg ha-1 and lowest seed yield was recorded in T8 (control) 1922.1 kg ha-1. The increase in yield due to growth regulators in groundnut might be due to an increase in distribution of peg and pod dry weight, higher partitioning of dry matter towards reproductive organs, increase in leaf thickness, number of pods per plant, higher no. of peg and total dry matter production (Faldu et al., 2018).
3.7 Haulm Yield
Among the treatments, significantly maximum haulm yield per plant was observed in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 45.89 g followed by T4 (Nano chitosan encapsulated NAA @ 50 ppm) 41.72 g.
Minimum haulm yield per plant was observed in T6 (Nano chitosan encapsulated BAP @ 50 ppm) 29.94 g followed by T7 (Nano chitosan @ 1 %) 31.99 g. Dry matter is the end product of assimilates from source organs via a transport path to the sink organs. The potential growth rate and potential capacity to accumulate assimilates has been shown to be an important parameter that quantitatively reflects the sink strength of an organ (Faldu et al., 2018).
3.8 Harvest Index (HI)
Harvest index denotes the partitioning efficiency of any genotype. Groundnut being semi determinate growth habit, Harvest index is generally low due to overlapping of vegetative and reproductive growth stages.
No significant difference was observed among all treatments in HI. Maximum harvest index was recorded in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 41.36 % and minimum was observed in T8 (control) 34.61 %. A rise in harvest index could be attributed to the synchronized interaction between growth and developmental characteristics (Chande et al., 2021).
An experiment was conducted to study the influence of nano chitosan encapsulated growth promoters on morpho-physiological and yield attributes of groundnut (Arachis hypogaea L.). Significant variability was observed among eight treatments. At 60 DAS, the tallest plants were recorded in T2 (GA3 @ 50 ppm) with 34.67 cm height, while the highest number of branches (4.44) and stem diameter (1.87 mm) were found in T5 (Nano chitosan encapsulated GA3 @ 50 ppm). Although no significant differences were observed in SPAD chlorophyll meter readings and leaf area index (LAI), T5 consistently showed superior values. These findings suggest that nano chitosan encapsulated GA3 enhances vegetative growth in groundnut and has potential for yield improvement. Highest seed yield was observed in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 2533.2 kg ha-1 and lowest seed yield was recorded in T8 (control) 1922.1 kg ha-1. Significantly maximum haulm yield per plant was observed in T5 (Nano chitosan encapsulated GA₃ @ 50 ppm) 45.89g followed by T4 (Nano chitosan encapsulated NAA @ 50 ppm) 41.72 g. Minimum haulm yield per plant was observed in T6 (Nano chitosan encapsulated BAP @ 50 ppm) 29.94 g. Maximum harvest index was recorded in T5 (Nano chitosan encapsulated GA3 @ 50 ppm) 41.36 % and minimum was observed in T8 (control) 34.61 %.
LITERATURE CITED
Chande, K.B., Deotale, R.D., Mate, P.R., Kalamkar, V.B and Banginwar, A.D. (2021). Impact of different concentrations of chitosan in enhancement of yield and yield contributing parameters in groundnut.
Crop Outlook Report of Andhra Pradesh : Groundnut (June 2023- May 2024). Centre for A g r i c u l t u r e and Rural Development Policy Research, Guntur, India.
Emongor, V., 2007. Gibberellic acid (GA3) influence on vegetative growth, nodulation and yield of cowpea (Vigna unguiculata (L.) Walp.).
Faldu, T. A., Kataria, G. K., Singh, C. K., & Savaliya, H. (2018). Effect of plant growth regulators on dry matter production and yield attributes of groundnut (Arachis hypogaea L.) cv. GJG-9. International Journal of Chemical Studies, 6(3), 2852-2855.
FAO. 2024 FAOSTAT Statistical Database. Food and Agricultural Organization of the United States.
Gardner, F.P., Pearce, R.B. and Mitchell, R.L. (1988). Transport and partitioning. In: Physiology of Crop Plants.
Hong Yan, W and Yu, L.S. (2001). The effect of chitosan and sodium alginate on the growth and photosynthesis of soybean. Journal of North East Agricultural University, China, 8: 156-160.
Leite, V.M., Rosolem, C.A and Rodrigues, J.D. (2003). Gibberellin and cytokinin effects on soybean growth. Scientia Agricola, 60. 537-541.
Ozkurt, N. and Bektas, Y. (2022). Alleviation of salt stress with chitosan foliar application and its effects on growth and development in tomato (Solanum lycopersicum L.). Türkiye Tarımsal Araştırmalar Dergisi. 9: 342-351.
Patil, M.B. (2019). Response of groundnut (Arachis hypogaea) to the exogenous application of growth hormones (IAA and GA₃). Journal of Bioscience and Agriculture Research. 22(01): 1829-1834.
Saini, C., Jain, N.K and Mathukia, R.K. (2016). Effect of sulphur and plant-growth regulators on growth, yield and economics of summer groundnut (Arachis hypogaea). Indian Journal of Agronomy, 61(1): 115-118.
Surendar, K.K., Vincent, S., Vanagamudi, M and Vijayaraghavan, H. (2013). Plant growth regulators and nitrogen responses on improving nutrient content of black gram (Vigna mungo L.). Plant Gene and Trait, 4(1).
Williams, R. (1946). The physiology of plant growth with special reference to the concept of net assimilation rate. Annals of Botany, 10(37): 41-72.
- Effect of Sowing Window on Nodulation, Yield and Post – Harvest Soil Nutrient Status Under Varied Crop Geometries in Short Duration Pigeonpea (Cajanus Cajan L.)
- Nanotechnology and Its Role in Seed Technology
- Challenges Faced by Agri Startups in Andhra Pradesh
- Constraints of Chcs as Perceived by Farmers in Kurnool District of Andhra Pradesh
- Growth, Yield Attributes and Yield of Fingermillet (Eleusine Coracana L. Gaertn.) as Influenced by Different Levels of Fertilizers and Liquid Biofertilizers
- Consumers’ Buying Behaviour Towards Organic Foods in Retail Outlets of Ananthapuramu City, Andhra Pradesh

