Impact of Drought Stress on Photosynthetic Pigments And Leaf Epicuticular Wax in Pigeonpea
0 Views
ABSTRACT
An experiment was carried to evaluate the performance of eighteen pigeonpea genotypes under drought stress. Drought stress was imposed at two phenophases of growth such as flowering and pod maturation stages. The parameters such as Chlorophyll a, b, a : b ratio, soluble protein, leaf epicuticular wax (ECW) and yield were recorded at flowering and pod maturation stages. Drought stress reduced the chlorophyll content and yield of pigeonpea. The drought tolerant genotypes showed significantly higher photosynthetic pigments, soluble protein, ECW and yield. These parameters can be used as reliable indices for selection in pigeonpea breeding for drought tolerance.
KEY WORDS:
Drought, photosynthetic pigments, soluble protein, ECW, yield
INTRODUCTION
Pulses are the important sources of protein for majority of population in India. The per capita per day availability is about 50 g as against the requirement of 85g recommended by the Nutritional Advisory Committee. Amongst the pulses, pigeonpea (Cajanuscajan) is widely consumed due to high protein content (typically 22% in dhal). It is a low input crop and usually grown under marginal environments that are often subjected to water stress at different stages of growth and development. Even for short-duration varieties, yield gets affected due to water stress during late flowering and early pod development stages (Lopez et al., 1996). During seed hardening, the crop requires considerable amount of water and at this crucial stage, water unavailability often causes terminal drought. Despite having a deeper root system, drought is still one of the major yield-limiting factors, especially at critical seedling and reproductive stages of pigeonpea (Saxena, 2008). Also, drought can occur at any phase of plant growth and the intensity of yield loss depends on the onset time, intensity and duration of stress (Hu and Xiong, 2014). Hence, there has been a rousing progress made in developing drought-tolerant pigeonpea genotypes. The present investigation was made to evaluate the genotypic differences in drought tolerance and quantify the loss in yield.
MATERIAL AND METHODS
The experiment was conducted at Millet Breeding Station, Tamil Nadu Agricultural University,Coimbatore with three replications in randomized block design. 18 genotypes were taken up for the study and the experimental plot was laid out with a size of 6.0 × 4.0 m2 . Spacing of 60 cm × 30 cm (ICPL genotypes) and 90 cm × 30 cm (Other genotypes) were adopted. The genotypes such as COPH 2, VRG 11, VRG 17, VRG 54, VRG 61, VRG 62, JKM 144, JKM 185, CO 5, VBN 2, ICPL 4777, ICPL 6997,ICPL 11119, ICPL 11175, ICPL 12755, ICPL 12974, ICPL 11375 and ICPL 11038 were taken up for the study.Recommended package of practices for red gram were followed. Drought stress was imposed to the genotypes by with holding the irrigation at flowering and pod development stages. The plants are maintained at irrigated (T1) and non-irrigated (T2) conditions in separate plots. Sampling was done at vegetative(S1),flowering (S2) and pod development (S3) stages.
Chlorophyll ‘a’, ‘b’ and a : b ratio were estimated in a fully expanded young leaf at specified time intervals and expressed in mg g-1 fresh weight basis (Yoshida et al.,1971). The soluble protein content was estimated fromleaf samples following the method of Lowry et al. (1951) and expressed as mg g-1 fresh weight basis. The KPI cuticular waxes content was estimated by using potassium dichromate and expressed in 1 g cm-2 (Ebercon et al.,1977). The seed yield was assessed at the time of harvest. At the time of harvest, the number of pods produced per cluster was counted and the mean was calculated and expressed in number per plant. Seed yield per hectare was calculated from the mean seed yield plant-1 and expressed in kg ha-1.
RESULTS AND DISCUSSION
Chlorophyll a and Chlorophyll b content (mg g-1)
Data on chlorophyll a content (Table 1) revealed that under stress conditions, high content were observed in COPH 2, ICPL 12755and ICPL 12974 whereas, the low values were observed in ICPL 6997, CO 5 and ICPL 4777 at both the stages. The chlorophyll b content (Table 1) was higher in drought tolerant genotypes (67.21 per cent) as compared to drought susceptible genotypes. The reductions in chlorophyll b contents due to stress were 14.25 to 28.27 per cent during S2 stage and 10.36 to 25.93 per cent during S3 stage.
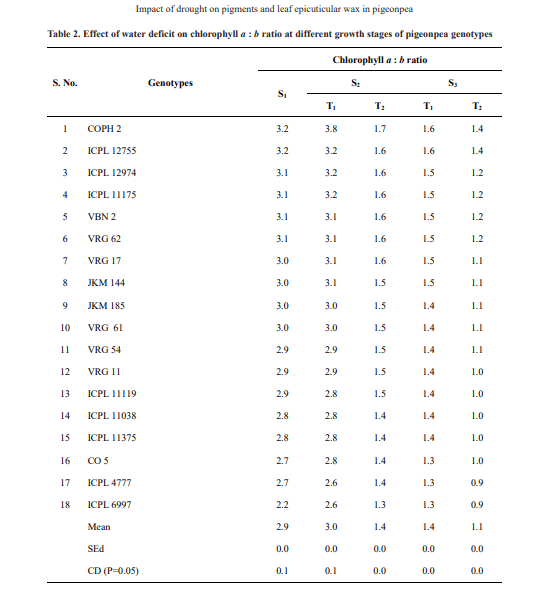
Chlorophyll a : b ratio
Data on Chlorophyll a : b ratios are presented in Table 2. The genotypes COPH 2, ICPL 12755 and ICPL 12974 recorded higher a: b ratios compared to other genotypes. During S2 and S3 stages, the genotypes COPH 2, ICPL 12755 and ICPL 12974 recorded lesser reductions in a: b ratio, whereas CO 5, ICPL 4777 and ICPL 6997 recorded larger reductions. The percent reduction in a: b ratio varied from 15.65 to 28.17 per cent and 7.29 to 32.39 per cent in S2 and S3 phases respectively.
Water stress caused a sharp decline in the chlorophyll a content, the chlorophyll b content, and the total chlorophyll content in sunflower varieties (Manivannan et al., 2007), chickpea (Mafakheri et al., 2010) and soybean (Makbul et al., 2011). The reduction in chlorophyll under drought stress is mainly due to the damage of chloroplasts caused by active oxygen species and the loss is associated to environmental stress and the variation in photosynthetic pigments.
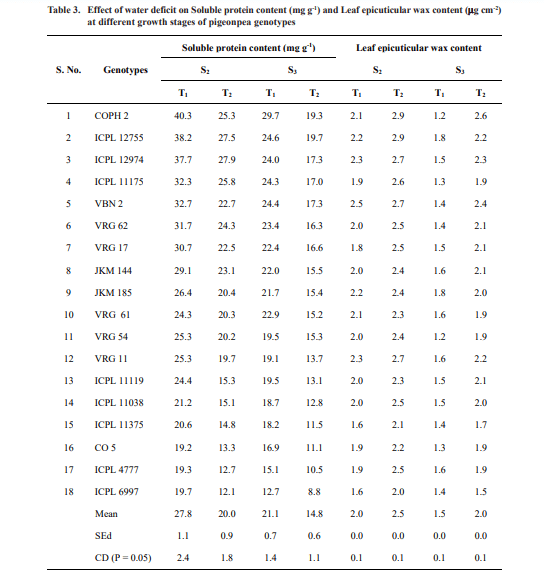
Soluble protein (mg g-1)
Data on soluble protein (Table 3) revealed that high amounts of soluble protein were observed in drought tolerant genotypes COPH 2, ICPL 12755 and ICPL 12974 to the extents of 25.3, 27.5 and 27.9 mg g-1 respectively during flowering stage. The soluble protein contents were low in drought susceptible genotypes CO 5, ICPL 4777 and ICPL 6997 with 13.3 12.7 and 12.1 mg g-1 respectively. At pod maturation stage, ICPL 12755 showed higher soluble protein content (19.7) and ICPL 6997 showed lower soluble protein content (8.8) and the percent reduction due to stress ranged from 19.82 to 36.81. The drought tolerant genotypes showed higher amount of soluble protein content even under water deficits. Generally, stress induces the production and the accumulation of ROS that causes oxidative damage at cellular level, disrupts cellular membranes, and leads to enzyme inactivation, protein degradation, and ionic imbalance in plants (Baier et al., 2005). In order to mitigate the negative effects of environmental stresses, plants increase the production of numerous compatible osmolytes, such as proline, glycine betaine, amines, and soluble sugars. Such compounds assist in imparting tolerance in stressed plants by creating osmotic balance, membrane integrity, enzyme and protein stability, and ROS detoxification (Blum, 2017).
Leaf Epicuticular Wax (mg cm-2)
Data on leaf epicuticular wax (Table 3) revealed that leaf epicuticular wax is one of the traits related to drought resistance. Drought stress caused an increase in wax content by 22.3 per cent and 40.2 per cent at flowering and pod maturations stages respectively. The leaf epicuticular wax contents were higher in drought tolerant genotypes with 21.42 per cent mean increase compared to drought susceptible genotypes. Biosynthesis of cuticular wax on the surfaces of the aerial plant parts is strictly associated with an adaptive response to water stress (Lee and Suh, 2013). Similar increase in wax content was also reported by Shardendu et al. (2011) in cowpea.
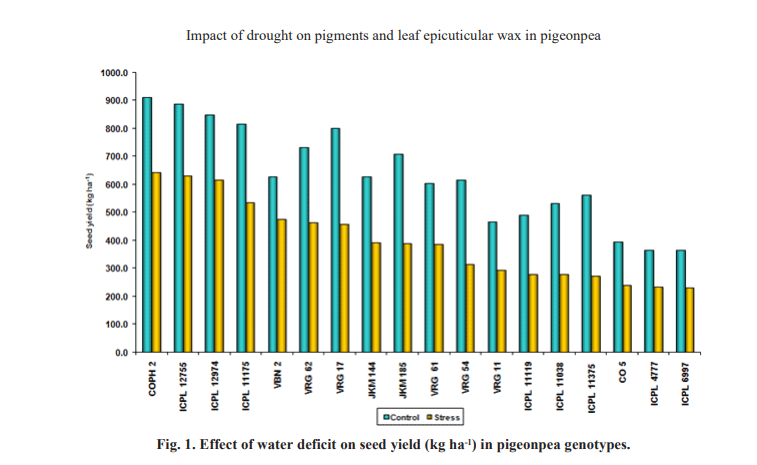
Seed Yield (kg ha-1)
Seed yield (Fig.1) is the product of many growth processes occurring through the development of the plant. In the present study, highest seed yield was observed in COPH2 and lowest in ICPL 6997. The genotypes ICPL 12755, ICPL 12974 exhibited reductions of 25.70 and
7.31 per cent respectively, but the susceptible genotypes CO 5, ICPL 4777 and ICPL 6997 showed reductions of 43.17, 41.16 and 39.37 per cent Yield reduction during stress might be due to lack of phenology development during early stages of crop growth. Reduced photosynthesis and decreased translocation of assimilates to the grain during drought result in lower grain weight and produce more empty grains (Liu et al., 2008). The decrease in yield might be due to less translocation of
assimilates to the developing pods of pigeon pea genotypes.
In conclusion, the present study revealed the differential tolerance limits in these genotypes for water deficit during important plant growth stages. Based on the findings the genotypes COPH 2, ICPL 12755, ICPL 12974, ICPL 11175, VBN 2 and VRG62 are grouped as drought tolerant while VRG 17, JKM 144, JKM 185, VRG 61, VRG 54 and VRG 11 as moderately drought tolerant genotypes while, ICPL 1119, ICPL 11038, ICPL 11375, Co 5, ICPL 4777 and ICPL 6997 as drought susceptible genotypes.
LITERATURE CITED
Baier, M., Kandlbinder, A., Golldack, D and Dietz, K. 2005. Oxidative stress and ozone: perception, signalling and response. Plant Cell and Environment. 28: 1012-1020.
Blum, A. 2017. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant, Cell and Environment. 40: 4-10.
Ebercon, A., Blum, A and Jordan, W.R. 1977. A rapid colorimetric method for epicuticular wax content of sorghum leaves. Crop Science. 17: 179-180.
Lee, S.B and Suh, M.C. 2013. Recent advances in cuticular wax biosynthesis and its regulation in Arabidopsis. Molecular Plant. 6: 246-249.
Liu, K., Ye, Y., Tang, C., Wang, Z and Yang, J. 2008. Responses of ethylene and ACC in rice grains to soil moisture and their relations to grain filling. Frontiers of Agriculture in China. 2(2): 172-180.
Lopez, F.B., Johansen, C and Chauhan, Y.S. 1996. Effects of timing of drought stress on phenology, yield and yield components of short-duration pigeon pea. Journal of Agronomy and Crop Science. 177: 311- 320.
Lowry, O.H., Rosebrough, Farr, L.A and Randall, R.J. 1951. Protein measurement with folin phenol reagent. Journal of Biological Chemistry. 192: 265- 275.
Nagajothi et al.,
Mafakheri, A., Siosemardeh, A., Bahramnejad, B., Struik,
P.C and Sohrabi, Y. 2010. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Australian Journal of Crop Science. 4: 580-585.
Makbul, S., Saruhan Güller, N., Durmuº, N and Güven,
S. 2011. Changes in anatomical and physiological parameters of soybean under drought stress. Turkish Journal of Botany. 35: 369-377.
Manivannan, P., Jaleel, C.A., Sankar, B., Kishorekumar, A., Somasundaram, R., Alagu Lakshmanan, G.M and Panneerselvam, R. 2007. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids and surfaces B: Biointerfaces. 59: 141-149.
Saxena, K.B. 2008. Genetic improvement in pigeon peas: a review paper. Journal of Tropical Plant Biology. 1: 159-178.
Shardendu, K.S and Raja Reddy, K. 2011. Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (Vigna unguiculata [L.] Walp.) under drought. Journal of Photochemistry and Photobiology B: Biology. 105: 40-50.
Yoshida, S., Forno, D.A and Cock, J.H. 1971. In: Laboratory manual for physiological studies of rice. IRRI, Philippines. 36-37.
- Genetic Divergence Studies for Yield and Its Component Traits in Groundnut (Arachis Hypogaea L.)
- Correlation and Path Coefficient Analysis Among Early Clones Of Sugarcane (Saccharum Spp.)
- Character Association and Path Coefficient Analysis in Tomato (Solanum Lycopersicum L.)
- Survey on the Incidence of Sesame Leafhopper and Phyllody in Major Growing Districts of Southern Zone of Andhra Pradesh, India
- Effect of Organic Manures, Chemical and Biofertilizers on Potassium Use Efficiency in Groundnut
- A Study on Growth Pattern of Red Chilli in India and Andhra Pradesh

