IDENTIFICATION OF RESISTANT SOURCES AGAINST HORSE GRAM YELLOW MOSAIC DISEASE IN HORSE GRAM
0 Views
B. SUSHMA*, PRADEEP MANYAM, P. SRI VALLI, M. KRISHNA REDDY AND ESWAR RAMI REDDY
Department of Plant Pathology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
Horse gram Yellow Mosaic Disease (HgYMD) is one of the major limiting factor for Horse gram production in India which
may cause complete yield loss. Thirty seven genotypes of Horse gram were screened in a Randomized Complete Block Design (RCBD) with three replications under natural disease epiphytotic conditions during rabi, 2022. A total of eighteen horse gram genotypes exhibited resistant reaction with low Percent Disease Incidence with a maximum of 0.7 %. Among which, AVTH-12 have shown highly resistant reaction. The apparent rate of infection which depicts disease progression was calculated and found gradual increase in disease in all susceptible genotypes viz., HG-17-1, BSP21-7, BSP21-4, BSP21-3, Indira Kulthi-1, BSP21-5, Bilasa, BSP21-11.
KEYWORDS: Horse gram Yellow Mosaic Disease, Screening, Genotypes, Management and Horse gram Yellow Mosaic Virus.
INTRODUCTION
Horse gram [Macrotyloma uniflorum (Lam.)] Verde. Syn. Dolichos biflorus is a hardy legume popularly known as poor man’s pulse crop, for its easily digestible quality protein and commonly known as kulthi, one of the drought tolerant crop grown in peninsular India. It belongs to the family Leguminosae and sub-family Papilionaceae. Horse gram is a perennial climbing plant with rhizome, growing to a height of 60 cm bears pods which are short and hairy. It has more advantages like adaptability to poor soil, adverse climatic conditions and improve the soil fertility by fixing atmospheric nitrogen and increasing the organic matter of soil. It occupies important place among pulses because of its ability to resist severe drought conditions. It is the only choice crop of the farmers for delayed sowing due to late receipt rains. It is widely used as cattle feed for its valuable protein (23-30%) and vitamins and also has medicinal properties and hence used in treatment of kidney and gall bladder stone, bronchitis, cough, cold and urinary diseases (Thakur, 1979; Khedar et al., 2008).
Since it is a hardy drought resistant plant, it has been cultivated as a low input agricultural crop in the marginal lands. It is cultivated in both the seasons (rabi and kharif) in different parts of the country. It is grown generally as a main crop, mixed crop with ragi and relay crop with maize, jowar and ragi (Barnabas et al., 2009).
The productivity of Horse gram is affected by many fungal and viral diseases. Among viral diseases, Yellow Mosaic Disease (YMO) is one of the major constraints for its cultivation in peninsular India and was first observed in southern districts of Karnataka.
Yellow mosaic disease (YMD) incidence on pulses lead to substantial yield losses ranging from 50- 100% based on the stage of the crop, genotype, vector population, weather factors etc. (Fauquet et al., 2003 and Maruthi et al., 2006). YMD is a severe disease in summer and late rabi seasons that affect pulse crops caused by whitefly transmitted begomoviruses belonging to the Geminiviridae family. The disease causes yellow discoloration on the leaves that leads to irregular, small, greenish yellow mosaic symptoms. Severe infection leads to stunted growth of the plant and reduction in the leaf size (Muniyappa et al., 1976 and Prema, 2013).
The rapid spread of the YMD due to increase in Bemisia tabací population results in almost complete loss of the crop during summer (Muniyappa et al., 1978).
Although vector management by insecticide sprays is one of the effective management strategies for viral disease management, it is not economical and environmental friendly. The present experiment was conducted for identification of resistant sources and using them in breeding programme for the development of resistant varieties will be effective.
MATERIAL AND METHODS
Experimental Design
The genotype screening experiment against YMD was conducted during late rabi (February-April), 2022 under natural field conditions at S.V. Agricultural College Tirupati, Andhra Pradesh when the vector population and natural incidence of YMD is naturally high. The field was sown according to RCBD with 3 replications. Each genotype was sown in 3 meter row (each entry 2 lines) with a spacing of 30 × 5 cm (120 plants in each replication). A local variety (CHRG- 19) which was observed to be highly susceptible in the previous two seasons among farmer’s fields was used as check. Susceptible entry was sown after every four lines and sown around the borders to naturally increase the disease pressure.
Sources of Genotypes
A total of 37 gentotypes consisting of germplasm, advanced breeding lines and cultivars obtained from (IGKV, TCB college of Agriculture and Research station, Chhattisgarh (Eighteen) RARS (ANGRAU), Tirupati (Six) and ARS (ANGRAU), Rekulakunta (Thirteen) were evaluated to identify resistant sources for YMD.
Natural screening
The crop was raised according to standard cultivation practices. No chemicals have been used to allow disease development to it’s full potential. After 45 DAS three readings were recorded for every 15 days interval, from each genotype 5 plants were taken randomly and disease incidence was scored based on 1-9 arbitrary scale (Table. 1). The per cent disease index was calculated by using the formula.
Per cent Disease Index =
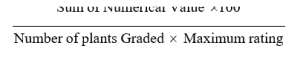
The genotypes were categorized into highly resistant, Resistant, Intermediate resistant, and susceptible based on gradings (Table 2).
Apparent rate of infection (r)
Speed, at which an epidemic develops, is called the apparent rate of infection (r). The disease index data was recorded at 15 interval (45, 60 and 75) to calculate the apparent rate of disease development using the formula suggested by Vander Plank (1968), where r is the apparent rate of infection in non-logarithmic phase, X1 and X2 symbolizes the percent disease index at time t1
and subsequent fifteen days time t2. (Table 5)
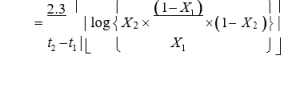
Table 1. The rating scale for scoring horse gram yellow mosaic disease (Alice and Nadarajan, 2007)
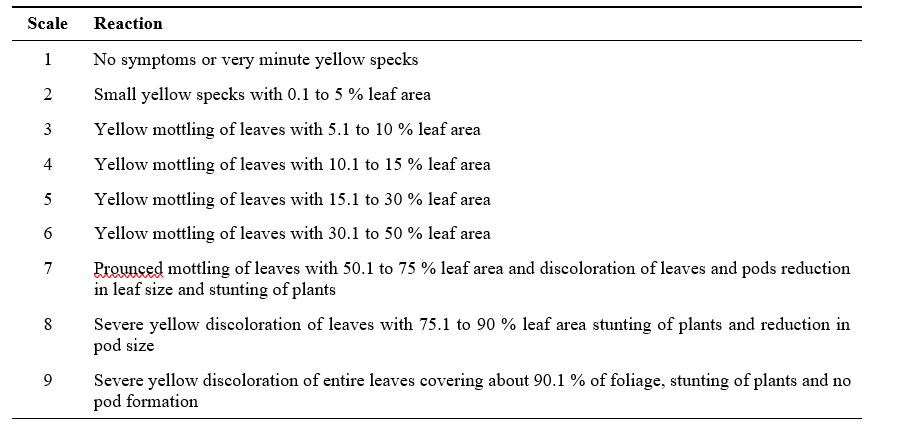
Table 2. Disease reaction scoring scale for Horse gram yellow mosaic virus in horse gram.

RESULTS AND DISCUSSION
Screening under natural disease epiphytotic condition
From the field evaluation of 37 genotypes the PDI values are ranged upto 97.20 (Table 3). A total of eighteen genotypes (BSP21-9, BSP21-8, C.G Kulthi-3, Acc. No: 68599, BSP21-1, BSP21-2, AVTH-4, AVTH- 5, AVTH-6, AVTH-11, BSP-17-1, AVTH-12, AVTH- 8, Acc. No: 71764, Acc. No: 139544, Acc. No: 120785, Acc. No: 71808, Acc. No: 139541) have shown resistant reaction to YMD (Fig. 1). The PDI observed among resistant ranged between 0- 0.7%. Only five genotypes exhibited to be moderately resistant (Acc. No: 277570, HG-11-1, Acc. No: 71768, C.G Kulthi -2, Acc. No:139538) for which, 1.83-5.20% of PDI was observed. Whereas six genotypes (BSP19-2, Acc. No: 139498, HG-25-1, BSP21-7, BSP19-3, BSP-17-3) falls under intermediate resistance. The rest eight genotypes showed varied susceptible reaction such as HG-17-1, BSP21-7, BSP21-4, BSP21-3, Indira Kulthi-1, BSP21-5, Bilasa and BSP21-11. (Table 3 and 4). The CRHG-19 showed 100% infection.
An increase in the infection rate of HgYMD growth was observed in all susceptible genotypes, so that the chances of epidemics is more in almost all the susceptible genotypes of Horse gram.
The apparent rate of infection in susceptible genotypes was observed in Bilasa (0.453), Indira Kulthi (0.45), BSP21-3 (0.20) and BSP21-5 (0.454) indicates the disease has epidemic rate of infection.
Rajkumar et al. (2009) screened five hundred horse gram genotypes against Horse gram Yellow Mosaic Virus under field conditions. Out of five hundred genotypes only seven genotypes viz., AK-21, AK-34, AK-38, AK-26, DPI-2278, Tcr-512 and AK-36 showed resistant reaction to HgYMD. Similarly Parimala et al. (2011) evaluated 23 genotypes for YMV, the genotypes HG-75, HG-63, HG-52, HG-59, HG-14, and AK-38, were free from infection. Seventeen genotypes exhibited moderately resistant reaction to YMV.
Durga et al. (2014) screened 23 horse gram accessions against YMV and wilt during rabi, 2010- 2011. The straw coloured accessions were highly resistant (0.55 %) than light straw coloured (1.19 %) and black coloured (2.52 %) accessions. Only one genotype HG 35 was susceptible to YMV remaining all shows resistant. Likewise Prema et al. (2017) also screened one hundred and ten germplasm lines against HgYMD during 2012. The disease incidence was ranged from 4.34-94.73 percent. Out of these five genotypes shows highly resistant reaction, three genotypes shows resistant reaction, two genotypes shows moderately resistant, ten genotypes shows susceptible reaction remaining eighty eight genotypes showed highly susceptible reaction.
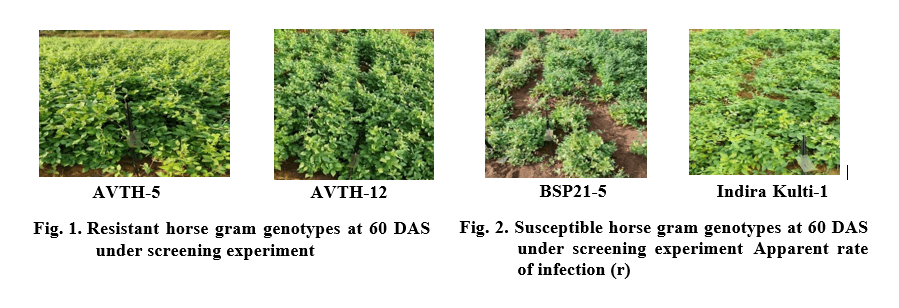
Table 3. Screening of horse gram genotypes against HgYMD resistance
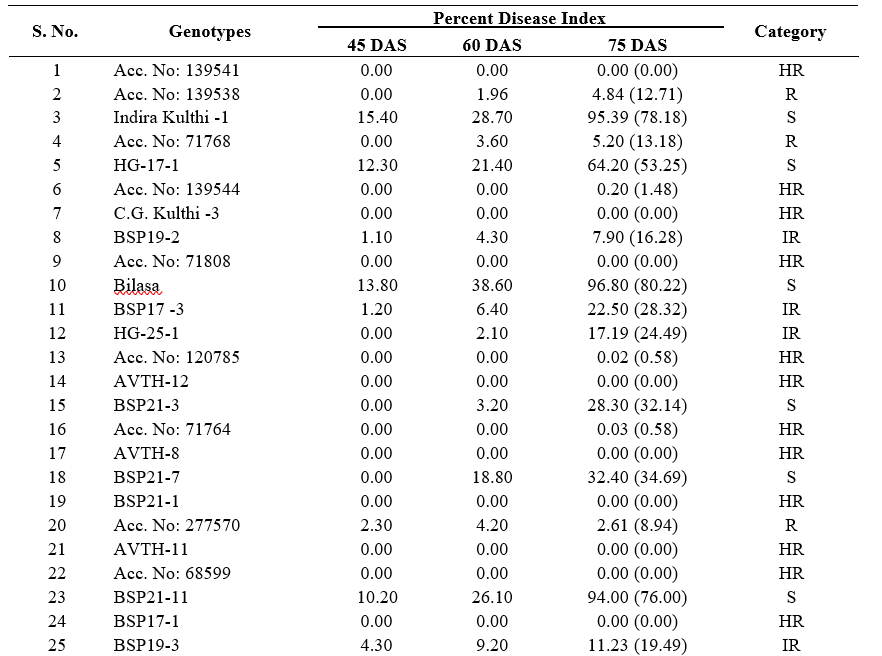
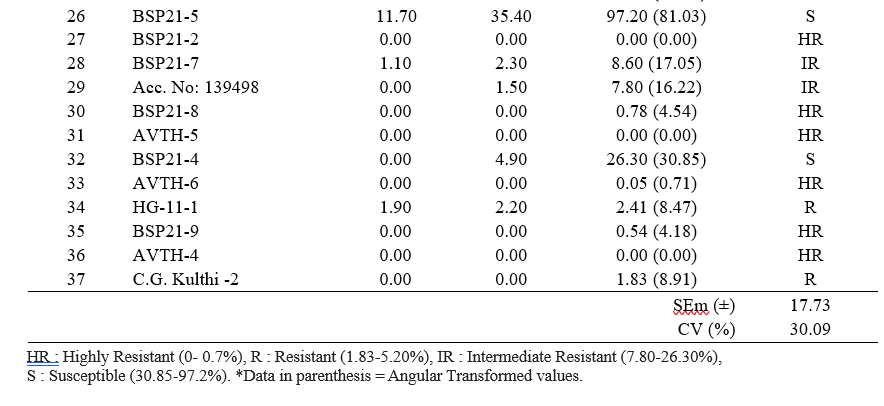
Table 4. Grouping of horse gram genotypes based on degree of resistance against Horse gram Yellow Mosaic Disease (HgYMD)
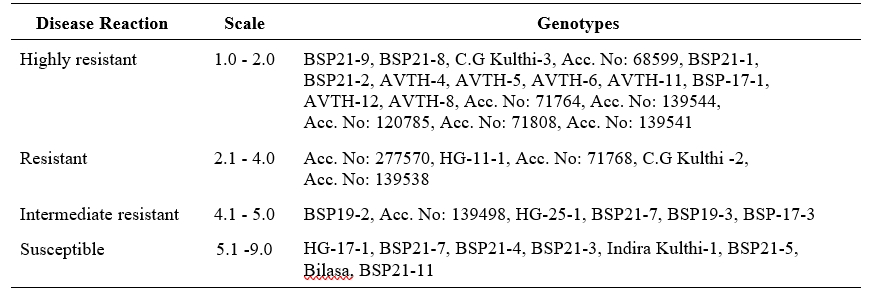
Table 5. Apparent rate of infection (r) of Horse gram genotypes for HgYMD
Disease resistance evaluation for genotypes is a crucial step in controlling of plant diseases. Resistant genes can be identified through routine screening procedures such as evaluation of genotypes to a certain extent. Identification of resistant lines is essential in the field of integrated disease management which is an important concept in the agriculture. Earlier studies indicated that identification of resistant sources to YMV is a reliable option for controlling YMD. However, critical investigations are necessary to establish the resistance level, in the genotypes and to further confirm them to finally include in breeding programmes.
A total of eighteen Horse gram genotypes exhibited resistant with low PDI in which they can be useful for developing resistant varieties in breeding programmes. The apparent rate of infection which depicts disease progression was calculated and found gradual increase in disease in all susceptible genotypes viz., Bilasa (0.453), Indira Kulthi (0.45), BSP21-3 (0.20) and BSP21-5 (0.454) was observed.
LITERATURE CITED
Alice, D and Nadarajan, N. 2007. Screening techniques and assessment methods for disease resistance. Department of Pulses. TNAU.
Barnabas, A.D., Radhakrishnan, G.K and Usha, R. 2009. Characterization of a begomovirus causing Horse gram yellow mosaic disease in India. European Journal of Plant Pathology. 127: 41-51.
Das, A., Aghora, T.S., Reddy, M.K., Nandeesha, P and Venugopalan, R. 2019. Identification of source of resistance to Horse Gram Yellow Mosaic Disease (HgYMD) in French Bean (Phaseolus vulgaris L.). Legume Research-An International Journal. 1: 6.
Durga, K.K., Varma, V.S and Reddy, A.V.V. 2014. Sources of resistance to wilt and ymv in horse gram. Journal of Global Biosciences ISSN. 3(1): 280-284.
Fauquet, C.M., Bisaro, D.M., Briddon, R.W., Brown, J.K., Harrison, B.D., Rybicki, E.P., Stenger,
D.C and Stanley, J. 2003. Revision of taxonomic criteria for species demarcation in the family Geminiviridae, and an updated list of begomovirus species. Archives of virology. 148(2): 405-420.
Khedar, O.P. 2008. Pulses: Status and cultivation technology. Aavishkar Publishers. Jaipur.
Maruthi, M.N., Manjunatha, B., Rekha, A.R., Govindappa, M.R., Colvin, J and Muniyappa, V. 2006. Dolichos yellow mosaic virus belongs to a distinct lineage of Old World begomoviruses; its biological and molecular properties. Annals of Applied Biology. 149(2): 187-195.
Muniyappa, V., Reddy, H.R and Ali, M. 1978. Studies on the yellow mosaic disease of horse gram [Dolichos biflorus], IV. Epidemiology of the disease [India]. Mysore Journal of Agricultural Sciences.
Muniyappa, V., Reddy, H.R and Shivashankar, G. 1976. Studies on the yellow mosaic disease of horse gram (Dolichos biflorus Linn.). II. Host range studies. Mysore Journal of Agricultural Sciences.
Parimala, K., Meenakumari, K.V.S., Sudhakar, R and Kanaka Durga, K. 2011, Screening of horse gram genotypes against yellow mosaic virus and powdery mildew diseases. Indian Journal of Plant Protection. 39: 160.
Prema, G.U and Rangaswamy, K.T. 2017. Field evaluation of horse gram germplasm/genotypes against Horse gram Yellow Mosaic Virus (HgYMV) Disease and biological transmission of horse gramyellow mosaic virus to different leguminous hosts through white flies. International Journal of Agricultural Science. 0975-3710.
Prema, G.U., Rudraswamy, P and Rangaswamy, K.T. 2013. Field screening of horse gram (Macrotyloma uniflorum) genotypes against horse gram yellow mosaic virus (HGYMV) disease. BIOINFOLET-A Quarterly Journal of Life Sciences. 10(2b): 599-601.
Rajkumar, S.G., Prameela, H.A., Rangaswamy, K.T., Divya, B.L., Shankarappa, K.S., Viswanatha, K.P and Maruthi, M.N. 2009. Sources of resistance to Horse gram yellow mosaic virus disease. Journal of Plant Protection and Environment. 6(1): 86- 89.
Thakur, C. 1979. Horse gram. Scientific Crop Production. 1: 319-320.
Vanderplank, J.E. 1963. Plant diseases: Epidemics and control. Academic Press, New York. Plant diseases: Epidemics and control. Academic Press, New York.
- Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
- Brand Preference of Farmers for Maize Seed
- Issues That Consumer Experience Towards Online Food Delivery (Ofd) Services in Tirupati City
- Influence of High Density Planting on Yield Parameters of Super Early and Mid Early Varieties of Redgram (Cajanus Cajan (L.) Millsp.)
- Influence of Iron, Zinc and Supplemental N P K on Yield and Yield Attributes of Dry Direct Sown Rice
- Effect of Soil and Foliar Application of Nutrients on the Performance of Bold Seeded Groundnut (Arachis Hypogaea L.)

