GENOTYPIC VARIATION FOR ROOT TRAITS TO PHOSPHORUS DEFICIENCY IN GROUNDNUT (Arachis hypogaea L.)
0 Views
P. LATHA*, K.V. NAGA MADHURI, A.R. NIRMAL KUMAR, P. SUDHAKAR, R.P. VASANTHI AND K. JOHN
Institute of Frontier Technology, RARS, ANGRAU, Tirupati- 517 502, Andhra Pradesh, India.
ABSTRACT
A pot culture experiment was conducted to screen 20 groundnut genotypes for high root mining ability under phosphorus (P) deficient conditions. Significant genotypic variation was observed among genotypes under both P sufficient and P deficient treatments. The results of the study revealed that the mean shoot length and pod weight recorded high in P sufficient soils compared to P deficient soils whereas the mean anthocyanins, root length, root weight and shoot weight recorded high in P deficient soils compared to P sufficient soils. There were no significant differences among the genotypes for number of lateral roots. Anthocyanins recorded high in 1157A and 1398 and recorded low in TCGS 1275 and 1273. The genotypes TCGS 1398, 1275 and 1273 recorded high shoot length, number of lateral roots, and shoot weight. Root length recorded high in TCGS 1330 and 1157. Root weight recorded high in 1398 and 1273. Pod weight recorded high in TCGS 1073, 1350 and 1273. Hence it can be concluded that, TCGS 1273 can be identified as P efficient line based on high root mining traits, and pod yield whereas TCGS 1398, 1275 and 1330 can be identified as P efficient lines based on their high root mining traits, and moderate pod yields.
KEYWORDS:
Groundnut genotypes, P efficiency, anthocyanins, root traits, shoot biomass
INTRODUCTION
Phosphorus (P) is one of the most limiting nutrients in groundnut production. Groundnut crop grown on marginal lands under rainfed conditions, are poor in nutrients and in these lands, phosphorus (P) deficiency is one of the important cause for low productivity (Schachtman et al., 1998; Lynch and Brown, 2008), mainly in low input farming systems occupying 5.7 billion hectare (B ha.) of global land. Most of the P applied in the form of fertilizers gets adsorbed by the soil and is not available for utilization by plants. . Several adaptive traits are developed by plants for tolerance to P insufficiency, i e., enhanced root mining abilities to acquire more P by increasing contact area, solubilizing P by acid exudation, mycorrhiza activity, etc. P deficiency induces many changes in root morphology and architecture in groundnut. Correcting soil P deficiency with heavy application of P fertilizer can be a solution but it is not possible for poor farmers (Lynch, 1995). Since P is a relatively immobile soil nutrient (Haynes et al., 1991), plants need to cope with heterogeneous P distribution in soils. Hence, root spreading, root architecture and elongation of lateral roots become important factors in acquisition of P (He et al., 2003). To sustain growth in such limiting conditions plants, plants have evolved a number of developmental and metabolic responses to adapt both the internal Phosphate (Pi) status in planta and the external soil Pi availability. These responses include changes in root morphology and architecture, accumulation of anthocyanin, and increases in the synthesis and secretion of organic acids into the rhizosphere (which enhance the utilization of Pi from insoluble inorganic compounds (Raghothama, 1999; López-Bucio et al., 2002). To improve acquisition of mineral elements by the root system, it has been suggested that, development of high performing genotypes in terms of root traits might increase crop yields on low fertile soils (White et al., 2013; Lynch, 2011). Studies on evaluating the groundnut genotypes with respect to anthocyanins and root traits in relation to P response are meagre. In the present study, twenty groundnut genotypes were screened for variability in anthocyanins and root traits under P deficiency stress.
MATERIALS AND METHODS
The groundnut genotypes for the study were procured from Department of Plant Breeding and Genetics, Regional Agricultural Research Station, Tirupati. A pot culture experiment was conducted in kharif, 2016 at Regional Agricultural Research Station, Tirupati. The experiment was carried out in a completely randomized design (CRD) with 20 genotypes sown in two P treatments i.e., P sufficient (78.6 kg ha-1 of soil available P2O5) and P deficient (23.5 kg ha-1 of soil available P2O5) soils and each treatment replicated thrice. The genotypes were grown in plastic pots filled with P sufficient and P deficient soils and four groundnut seeds were laid in each pot. At 60 DAS, anthocyanin content was measured as described by Kim et al. 2003. At harvest, observations were recorded for root parameters viz., root length (PRL), number primary lateral roots, shoot length (SL), root dry weight (RDW) and shoot dry weight (SDW) and pod yields were recorded after drying of individual plants and expressed in g plant-1. The statistical significance of variance was assessed by Two Way Analysis of Variance (ANOVA) and critical difference values at 5% level of significance were calculated to compare mean values by GENSTAT software.
RESULTS AND DISCUSSION
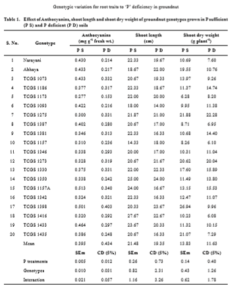
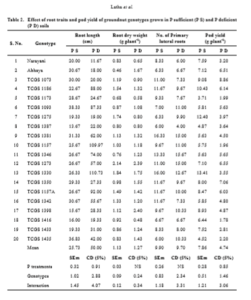
The results of the study to screen P efficient lines based on shoot and root morphology reveals that the mean shoot length, shoot biomass and pod weight significantly recorded high in P sufficient soils compared to P deficient soils whereas the mean anthocyanins, root length and root weight significantly recorded high in P deficient soils compared to P sufficient soils. There were no significant differences among the genotypes for number of lateral roots. The genotypes TCGS 1398, 1275, and 1273 significantly recorded high shoot length, number of lateral roots, and shoot weight in P deficient soils. Root length recorded high in TCGS 1330 and TCGS 1157 whereas root weight recorded high in 1398 and 1273 (table 1 and 2) in P deficient soils.
The visible accumulation of anthocyanin pigmentation is one of the characteristic responses of plants to Pi starvation. Anthocyanins and other polyphenolic compounds (e.g. flavonols and condensed tannins) have a wide range of functions in plants related to UV absorption, pathogen attack, and nutrient stress (Stewart et al., 2001; Kliebenstein, 2004). In the present study, anthocyanins recorded high in 1157A and 1398 and recorded low in TCGS 1275 and 1273 in P deficient soils.
To withstand P stress condition, plants acquire adaptations at physiological, biochemical and molecular levels. One of the adaptations under reduced P supply is changes in root morphology and these changes include, increased root shoot ratio, lateral root number, root length and biomass (Gahoonia and Neilson, 2004) to enhance P acquisition. In the present study, among the genotypes, TCGS 1398, 1275, and 1273 significantly recorded high shoot length, number of lateral roots, and shoot weight in P deficient soils. Root length recorded high in TCGS 1330 and TCGS 1157 whereas root weight recorded high in 1398 and 1273 (table 1 and 2) in P deficient soils. In the responsive genotypes, root growth is enormous to adapt with P stress condition, thus providing greater contact area, for better acquisition of less mobile element like P (Otani et al. 1996).
Increase in root-shoot ratio which is reported as significant change for adaptation to P deficiency in plants (Hammond and White, 2011) is due to increase in carbohydrates accumulation in roots. In the present study, TCGS 1273 recorded highest shoot and root biomass followed by TCGS 1398 and 1330. Genotypes with extensive root systems coupled with a large shoot system would be P efficient, contributing to yield stability during reduced P supply. An increase in root biomass in response to P stress might enhance P acquisition from the soil. Phosphorus efficient genotypes have usually highly branched root systems with numerous basal roots, while the inefficient plants had smaller, less branched roots (Hammond et al., 2009). P acquisition is strongly dependent on soil exploration and root architecture (Lynch and Beem, 1993). Therefore, genotypes having greater ability to tolerate P stress condition would be able to acquire P efficiently from low P soil. Phosphorus use efficient genotype showed higher relative root growth because of the additional P taken up by roots, allow further biomass accumulation which leads to more production. Phosphorus use efficient groundnut genotypes developed various adaptive strategies such as increased root length, root and shoot biomass and P content to enhance the P acquisition and utilization efficiency under P deficient soil condition (Amitkumar et al., 2009). One characteristic of plant Pi starvation response is simultaneous reduction in shoot growth and increase in root proliferation. The outcome of this response is the formation of a highly branched root system (associated with reduced primary root length, increased lateral root number, and density) and increases in both frequency and length of root hairs. These changes enhance the exploratory capacity of roots to search for Pi-rich patches present in the soil.
Genotypic variation for root traits to ‘P’ deficiency in groundnut
(Raghothama, 1999; Lynch and Brown, 2001). The present study also reveals that, shoot growth reduction and root growth increase in P deficient soils compared to P sufficient soils.
Pod yields varied significantly among the 20 groundnut genotypes (Nagamadhuri et al., 2019) under P insufficiency conditions. In the present study, pod yields recorded low in P deficient soils compared to P sufficient soils. Pod weight recorded high in TCGS 1073, 1350 and 1273 under P deficient conditions (Table 2).
The selected genotypes exhibited increased performance in various root traits and accumulated more root and shoot biomass under P insufficiency. The developed root system in P insufficient conditions would be responsible to enable plants to absorb more nutrients from the soil, thus increasing the shoot biomass. The increased performance of root traits was reflected in the acquisition of P and ultimately in the superiority of responsive genotypes.
In conclusion, genotypes differed for root traits and hence it can be concluded that, TCGS 1398, 1275 and 1330 can be identified as P efficient lines with high root mining traits but with moderated pod yields. These genotypes can be used as genetic material in plant breeding programmes for further improvement in pod yields. The genotype TCGS 1273 can be identified as P efficient line with high root mining traits, and also recorded high pod yields. Genetic basis of superiority in P deficient condition could be determined and genes responsible for P deficiency tolerance can be transferred to agronomically superior genotypes.
ACKNOWLEDGMENT
The author would like to thank Acharya N G Ranga Agricultural University for their financial support to conduct the study.
LITERATURE CITED
Amitkumar, Kusuma, P and Gowda, M.V.C. 2009. Genotypic variation for root traits in groundnut germplasm under phosphorus stress conditions. Journal of SAT Agricultural Research. 7.
Gahoonia, T.S and Nielsen, N.E. 2004. Barley genotypes with long root hairs sustain high grain yields in lowP field. Plant Soil. 262: 55-62.
Hammond, J.P and White, P.J. 2011. Sugar Signaling in Root Responses to Low Phosphorus availability. Plant Physiology. 156(3): 1033–1040.
Hammond, J.P., Broadley, M.R., White, P.J., King, G.J., Bowen, H.C., Hayden, R., Meacham, M.C., Mead, A., Overs, T and Spracklen, W.P. 2009. Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. Journal of Experimental Botany. 60: 1953-1968.
Haynes, B., Koide, R.T and Elliot, G. 1991. Phosphorus uptake and utilisation in wild and cultivated oats (Avena spp.). Journal of Plant Nutrition. 14: 11051118.
He, Y., Liao, H and Yan, X. 2003. Localized supply of phosphorus induces root morphological and architectural changes of rice. I. Split and stratified soil culture. Plant and Soil. 248: 247-256.
Lynch, J.P and Beem, J.J. Van. 1993. Growth and architecture of seedling roots of common bean genotypes. Crop Science. 33: 1253-1257.
Lynch, J. 1995. Root architecture and plant productivity. Plant Physiology. 109: 7.
Lynch, J.P and Brown, K.M. 2008. “Root strategies for phosphorus acquisition,” In White P. and Hammond J (eds). The Ecophysiology of Plant–Phosphorus Interactions. 83-116. Springer, Berlin.
Lynch, J.P. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology. 156: 1041–1049.
Otani, T., Ae, N and Tanaka, H. 1996. Phosphorus (P) uptake mechanism of crops grown in soils with low P status. II. Significance of organic acid in root exudates of pigeonpea. Soil Science and Plant Nutrition. 42: 553-560.
Schachtman, D.P., Reid, R.J and Ayling, S.M. 1998 Phosphorus uptake by plants: from soil to cell. Plant Physiology. 116: 447-453.
White J.P., Timothy, S., George., Lionel, X., Dupuy, Alison J., Karley, Tracy, A. Valentine., Lea Wiesel and Jane Wishart. 2013. Root traits for infertile soils. Frontiers in Plant Science. 4(10): 1-7.
Kim, J., Yi, H., Choi, G., Shin, B., Song, P.S and Choi, G. 2003. Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell. 15: 2399–2407
Raghothama, K.G. 1999. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molcular Biology. 50: 665–693.
Lynch, J.P and Brown, K.M. 2001. Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant Soil. 237: 225–237.
Nagamadhuri, K.V., Latha, P., Vasanthi, R.P., John, K., Reddy, P.V.R.M., Murali, G., Krishna, T.G., Naidu, T.C.M and Naidu, N.V. 2019. Evaluation of groundnut genotypes for phosphorus efficiency through leaf acid phosphatase activity. Legume Research. 42: 736-742.
Stewart, A.J., Chapman, W., Jenkins, G.I., Graham, I., Martin, T and Crozier, A. 2001. The effect of nitrogen and phosphorus deficiency on Xavonol accumulation in plant tissues. Plant Cell Environment. 24: 11891197.
Kliebenstein, D.J. 2004. Secondary metabolites and plant/ environment interactions: a view through Arabidopsis thaliana tinged glasses. Plant Cell Environment. 27: 675-684.
- Genetic Diversity Analysis of 64 Maize Inbred Lines for Yield Traits Using D2 Statistics and Principal Component Analysis
- Effect of Border Crops on Activity of Predatory Fauna In Blackgram (Vigna Mungo L.)
- Effect of Organic Nutrient Management Practices on Growth and Yield of Foxtail Millet
- Species Diversity of Sugarcane Shootborers in Major Sugarcane Growing Districts of Andhra Pradesh
- Faunistic Studies on Economically Important Lepidopterans in Different Field Crops of Tirupati District
- Production Potential of Sweet Corn as Influenced by Organic Manures and Foliar Nutrition

