Evaluation of Pseudomonas Fluorescens Against Pyricularia Oryzae in Vitro
0 Views
Rice blast caused by Pyricularia oryzae is one of the major destructive diseases of rice growing regions of the world including India. It causes yield loss up to 80 per cent (Ou, 1985) but normally can lead to 30 per cent yield loss annually (Talbot, 2003). P. oryzae is a hemi biotrophic pathogen, initially grow biotrophically and then switch to necrotrophic growth, killing the infected tissues. P. oryzae, however, invades foliar tissues biotrophically and necrotrophically simultaneously (Kankanala et al., 2007). The disease can strike all aerial parts of the plant. Most infections occur on the leaves, causing diamond-shaped lesions with a grey or white center to appear, or on the panicles, which turn white and die before being filled with grain.
Pseudomonas fluorescens is a gram negative, rod shaped, non pathogenic bacteria that colonize in soil, water and plant surface environments. Since they are well adapted in soil, P. fluorescens strains are being investigated extensively for use in bio control of pathogens in agriculture (Ganeshan and Kumar, 2006). Biological control of plant pathogens by antagonistic micro organisms is a potential non-chemical means and is known to be a cheap and effective eco-friendly method for the management of crop diseases (Cook and Baker, 1983). Many biocontrol agents from P. fluorescens and closely related species are well characterized for their ability to produce antimicrobial compounds, including 2, 4- diacetylphloroglucinol (DAPG), phenazines, hydrogen cyanide and surfactants (Haas and Defago, 2005).The use of biological control agents as an alternative to fungicides is increasing rapidly in the present day agriculture, due to the deleterious effects of chemical pesticides on human health and environment. Hence in the present investigation, Pseudomonas fluorescens isolates were tested for their antagonistic activity against P. oryzae.
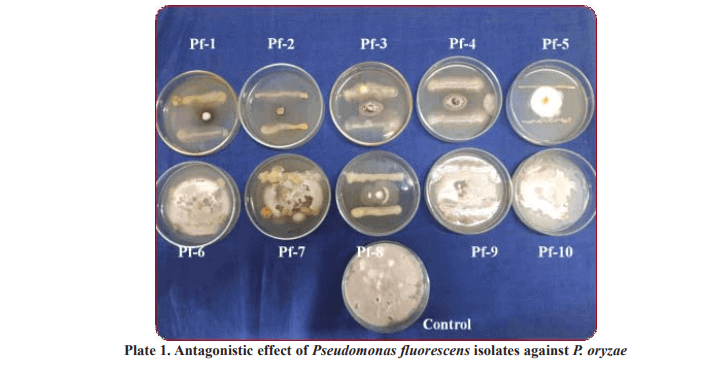
Two Pseudomonas fluorescens isolates were collected from Department of Plant Pathology, ARS, Nellore (Pf 1 and Pf 2) and eight isolates (Pf 3 to Pf 10) were from department of Agricultural microbiology, COA, Rajendranagar, PJTSAU. In order to find the antagonistic effect of Pseudomonas fluorescens against P. oryzae, in vitro, dual culture studies were employed (Mortan and Straube, 1955). 20 ml of PDA was poured aseptically into sterilized Petri dishes and allowed to solidify. Mycelial discs of 5 mm diameter from the edge of actively growing culture of P. oryzae placed on the periphery about 1 cm from the edge of the Petri dishes and Pseudomonas fluorescens was streaked on opposite side. Three replications were maintained for each interaction. The Petri dishes containing potato dextrose agar inoculated with P. oryzae (mono culture) alone served as control. All the Petri dishes were incubated at (25 ± 1°C) for fifteen days. At the end of incubation, the colony diameter of the P. oryzae was measured and the per cent inhibition of P. oryzae was calculated by adopting the formula given by Vincent (1927).
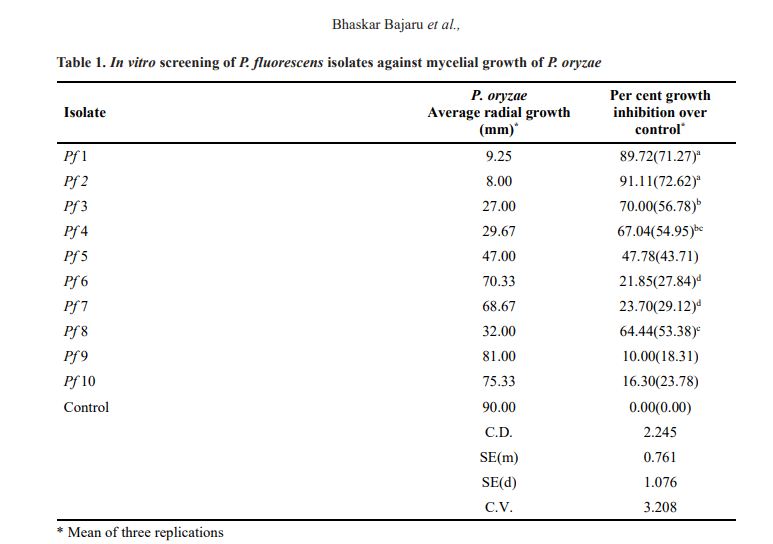
Results indicated that, the isolate Pf 2 had highest per cent inhibition of mycelial growth (91.11%) and it was closely followed by Pf 1 (89.72%). The isolates Pf 3, Pf 4 and Pf 8 showed the inhibitions at 70, 67.04 and
64.44 per cent, respectively. The mycelial inhibition was very low in case of the isolates Pf 9 (10.00%), Pf 10 (16.30%), Pf 6 (21.85%) and Pf 7 (23.70%) and did not sustain as evidenced by mycelial growth of P. oryzae which overgrew the above isolates in vitro in course of time (Table 1 and Plate 1).
The in vitro dual culture test is being used widely by researchers, to measure the mycoparasitic activity of Pf isolates against P. oryzae. This mycoparasitic activity of Pseudomonas spp. was well documented by Keel and Defago (1997). The results from the present study were
in confirmatory with the findings of many scientists who studied in vitro effect of Pseudomonas fluorescens against P. oryzae growth and reported the inhibition of 50 per cent (Goud and Muralikrishnan, 2008) and 59 per cent (Pandey and Chandel, 2014). Kulmitra et al., (2017) reported that some of Pseudomonas fluorescens did not show any inhibition of mycelial growth of P. oryzae as the pathogen over grew them. The inhibition of mycelial growth of the pathogen by Pseudomonas fluorescens may be due to the production of antibiotics. Production of antibiotis viz., HCN, pyrrolnitrin, phenazine and 2, 4- diacetyl phloroglucinol and lytic enzymes by P. fluorescens against fungal pathogens were reported by many workers (Ramamoorthy et al., 2001 and Saravanakumar et al., 2008).
Among ten Pf isolates, Pf 2 and Pf 1 had exhibited maximum antagonistic activity against P. oryzae, so that these two isolates can be used in future for management of rice blast disease at field level.
LITERATURE CITED
Cook, R.S and Baker, K.F. 1983. The nature and practice of biological control of plant pathogens. American Phytopathological Society, St Paul, Minn. 533.
Ganeshan, G and Kumar, M.A. 2006. Pseudomonas fluorescens a potential bacterial antagonist to control plant diseases. Journal of Plant Interactions. 1(3): 123-134.
Goud, M and Muralikrishnan, V. 2008. Biological control of three phytopathogenic fungi by Pseudomonas fluorescens isolated from rhizosphere. The Internet Journal of Microbiology. 7(2): 1-6.
Haas, D and Defago, G. 2005. Biological control of soil borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiology. 3: 307-319.
Kankanala, P., Czymmek, K and Valent, B. 2007.Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell. 19: 706-724.
Keel, C and Defago, G. 1997. Interactions between beneficial soil bacteria and root pathogens: mechanisms and ecological impact, In Multitrophic interactions in terrestrial systems (Gange, A. C and Brown, V. K. edition), 27-46. Blackwell Scientific Publishers, London, United Kingdom.
Kulmitra, A.K., Sahu, N., Kumar, V.B.S., Thejesha, A.G., Ghosh, A and Yasmin, G. 2017. In vitro evaluation of bio-agents against Pyricularia oryzae Cav. causing rice blast disease. Agricultural Science Digest. 37(3): 247-248.
Morton, D.J and Straube, W.H. 1955. Antagonistic and stimulatory effect of soil micro organisms upon Sclerotium rolfsii. Phytopathology. 45: 417-420.
Ou, S.H. 1985. Rice Disease. (2nd Edition), Common wealth Mycological Institute, Kew, Surrey, England. 201.
Pandey, S.K and Chandel, S.H.R. 2014. Efficacy of Pseudomonas as biocontrol agent against plant pathogenic fungi. International Journal of Current Microbiology and Applied Sciences. 3(11): 493-500.
Ramamoorthy, V., Viswanathan, R., Raguchandar, J., Prakasham, T and Samiyappan, R. 2001. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pest and diseases. Crop Protection. 20: 1-11.
Saravanakumar, D., Lavanya, N., Muthumeena, B., Raguchander, T., Suresh, S and Samiyappan, R. 2008. Pseudomonas fluorescens enhances resistance and natural enemy population in rice plants against leaf folder pest. Journal of Applied Entomology. 132(6): 469-479.
Talbot, N.J. 2003. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annual Review of Microbiology. 57: 177–202.
Vincent, J.M. 1927. Distortion of fungal hyphae in the presence of certain inhibitors. Nature. 159: 850.
- Genetic Divergence Studies for Yield and Its Component Traits in Groundnut (Arachis Hypogaea L.)
- Correlation and Path Coefficient Analysis Among Early Clones Of Sugarcane (Saccharum Spp.)
- Character Association and Path Coefficient Analysis in Tomato (Solanum Lycopersicum L.)
- Survey on the Incidence of Sesame Leafhopper and Phyllody in Major Growing Districts of Southern Zone of Andhra Pradesh, India
- Effect of Organic Manures, Chemical and Biofertilizers on Potassium Use Efficiency in Groundnut
- A Study on Growth Pattern of Red Chilli in India and Andhra Pradesh

