Efficacy of Native Isolates of Bacillus Thuringiensis Berliner on Mortality of 3rd Instar Larvae of Fall Army Worm, Spodoptera Frugiperda (J.E. Smith)
0 Views
T. JAYA BHARGAVI*, K.V. HARI PRASAD, K. DEVAKI AND K. JYOTHSNA
Department of Entomology, S.V. Agricultural College, ANGRAU, Tirupati
ABSTRACT
Four native isolates of Bacillus thuringiensis Berliner viz., V13, CF24, CFo76 and W85 (collected from soils from in and around different locations from Tirupati, Chittoor Dt., Andhra Pradesh, India) were tested at five different concentrations against third instar larvae of Fall Army Worm Spodoptera frugiperda (J.E. Smith). Among the four native isolates tested, V13 isolate showed highest per cent larval mortality (86.66%), Lowest values of LC50 (6.28 × 102 CFU/ml) and LT50 (69.02 h) for third instar larvae of FAW, indicating that the isolate V13 could be used as a potent Bt native isolate for the management of third instar larvae of S. frugiperda.
KEYWORDS: Bacillus thuringiensis, LC50, LT50, Native isolates, per cent larval mortality, Spodoptera frugiperda
INTRODUCTION
Maize is the third most important cereal crop after rice and wheat in India, sharing about 2 per cent the world’s maize production. About 71 per cent of maize in India is being produced during the kharif season in different states viz., Karnataka, Madhya Pradesh, Tamil Nadu, Maharashtra, Telangana, Uttar Pradesh and Rajasthan with Karnataka being the leader. Bihar, Andhra Pradesh and Tamil Nadu are the states in India that accounts to 40 per cent of maize crop in rabi season (Anonymous, 2017).
Among the biotic constraints that limit the maize production, insect pests viz., stem fly, stem borer, cornworm, aphids, shoot fly etc. are the major insect pests inflicting maximum yield loss. Fall ArmyWorm, Spodoptera frugiperda popularly known as FAW is native to tropical and subtropical Americas. FAW is a polyphagous pest which majorly prefers plants/crops belonging to Poaceae family and most commonly recorded in wild and cultivated grasses like maize, rice, sorghum and sugarcane (Anonymous, 2020). It has been reported that around 353 host plant species of 76 plant families viz., Poaceae (106), Asteraceae (31) and Fabaceae 2018).
Invasion and existence of S. frugiperda in India was confirmed by the University of Agricultural and
Horticultural Sciences, Shivamogga, Karnataka during May-June, 2018 and since then this insect has become a major threat to maize cultivation in India (Sharanabasappa et al., 2018). It has been reported that damage by this insect pest causes a three per cent reduction in grain yield (Lima et al., 2010) and with an annual loss up to US dollars 400 million in Brazil (Figueiredo et al., 2015). During the year, 2018 due to this pest in India, production of maize fell by 3.2 per cent equivalent to 27.8 million tons of grain.
Farmers rely predominately on the use of synthetic insecticides for managing this insect pest. Use of insecticides as a sole tool in management of insect pests has potential draw backs, that includes effect on the environment, non-target organisms and natural enemies coupled with outbreak of secondary pest and resurgence (Togbe et al., 2014). An attractive alternative tool for management of insect pests is the use of biological pesticides due to their ecofriendly and target selective characteristics (Ali et al., 2015) and microbial agent containing insecticides are considered as good replacement due to the absence of mammalian toxicity (Sabbour, 2003). Biopesticides like B.t, and Beauveria bassiana can provide an alternative and environment friendly option to control several important insect pests (Taggar et al.,2014).
Over 100 bacteria have been identified as insect pathogens, among them, B.t. has got maximum importance as a microbial agent (Muhammad et al., 2016). B.t is a gram positive, spore forming bacterium having insecticidal properties and is benign to natural enemies, quite safe to mammals and also environmentally acceptable (Ali et al., 2015). B.t produces crystal toxins that activates into protoxins in the insect gut due to alkaline pH of 9.0-9.5. due to insertion of protoxins on receptor proteins of midgut membrane, results in formation of pores in the gut resulting gut paralysis and septicemia causing the death of larvae.
MATERIAL AND METHODS
The research work was carried out at Insectary and Insect pathology laboratory, Department of Entomology,
S.V. Agricultural College, Tirupati during 2020 –21.
Rearing and maintenance of S. frugiperda
Rearing of S. frugiperda was done at the Insectary, Department of Entomology, S.V. Agricultural College, Tirupati at temperature of 25 ± 2°C, relative humidity of 75.00 ± 5.00 per cent and photoperiod of 12 h light / 12h dark. Eggs of S. frugiperda were collected from surrounding farmer’s maize fields and were kept in plastic troughs (200mm diameter and 100 mm height).First instar larvae were reared in groups on a corn flour based artificial diet, late instars were reared individually up to pupation (Barreto et al.,1999).
Larvae took 15-20 days to complete the larval duration and pupated in rearing troughs. The pupae were collected and were shifted to adult rearing cages (35 × 25 × 45 cm) provided with a maize seedling as oviposition substrate. A cotton swab dipped in 10 per cent honey solution was provided as food material for the emerging adults. Eggs laid by the adults on the maize seedlings were collected and the hatched larvae were reared on the artificial diet up to third instar (identified by change of instar, width of head capsule i.e., 0.81 to 0.95 mm and duration (4 to 6 days from hatching) and these third instar larvae were used for bioassay studies.
Culturing of B.t.
Forty ml of Luria Bertani broth was taken in 50ml conical flask and sterilized in autoclave at 121°C, 15 lbs pressure for 15 minutes. After cooling, the broth was inoculated with one loop of each native B.t isolate in different conical flasks. The flasks with culture broth were subjected to shaking for 48 h. Serial dilutions were prepared at ![]() dilutions and 100 μl of each dilution was taken and spread on Luria agar plate with ‘L shaped’ rod. The plates were kept in incubator for overnight at 37°C. After 24 h colony count was taken based on the standard formula. (Aneja, 2003).
dilutions and 100 μl of each dilution was taken and spread on Luria agar plate with ‘L shaped’ rod. The plates were kept in incubator for overnight at 37°C. After 24 h colony count was taken based on the standard formula. (Aneja, 2003).
The number of bacteria colony or CFU /ml =
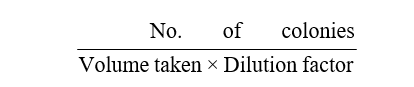
After colony counting, a standard concentration of 1.5 × 107 CFU/ml was prepared for all the isolates from which serial dilutions viz., 1.5 × 107; 106; 105; 104; 103 CFU/ml were made and used for the bioassay experiments.
Bioassay studies of B.t. against third instar larvae of frugiperda
Five grams artificial diet cubes were dipped in different dilutions of native B. t. isolates for 4 to 5 min then air dried. Ten third instar larvae of S. frugiperda were released on to the artificial diet and were allowed to feed on treated artificial diets. The experiment was replicated three times. Thirty larvae were tested for each dilution and a total of five concentrations were used for each isolate. Commercial formulation (Dipel) was also prepared into different concentrations and used as check. The diet treated with distilled water was served as control. Larval mortality was recorded after 24 h of treatment at regular intervals and continued to till pupation or death of the larvae. Per cent larval mortality was recorded by dividing number of larvae died out of total number of larvae treated.
Statistical analysis
The recorded larval mortality was converted into percentage values by using the following formulae and then transformed to arc-sine values. Mean values were separated by DMRT.
Per cent larval mortality =
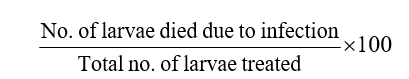
The larval mortality percentage data obtained from
B.t bioassay, were subjected to Probit analysis for determining LC25, LC50, LC75 and LC99 values with
the help of SPSS package.
RESULTS AND DISCUSSION
Among native isolates and commercial formulation tested, the mean larval per cent mortality recorded at different concentrations were presented in Table1.
Among the native isolates tested (at 1.5 × 107 CFU/ ml) the highest mortality was observed in V13 isolate (86.66%) followed by W85 isolate (73.33%) which were significantly different from each other. The larval mortality of V13 isolate (90.00%) was on par with Dipel (commercial formulation), whereas no mortality was recorded in the control treatment.
The present investigation revealed that the mortality has increased with an increasing in the concentration indicating a positive correlation between the mortality of the larvae and the dose applied. In accordance to the present study, Pavani (2019) evaluated 15 native B.t. isolates against third instar larvae of Spodoptera litura at different concentration and found that the mean percent larval mortality (which were in the range of 33.33 to 90.00%) was dose dependent.
In the present investigation though all the native isolates tested showed promising results (around 80.00% mortality against third instar larvae of S. frugiperda) the commercial formulation Dipel had shown highest mortality (90.00%) surpassing the native isolates, this probably is due to role of strain in the commercial formulation Dipel i.e., B.t kurstaki.
Nethravathi and Huger (2010) also investigated the efficacy of B.t isolates against five-day-old larvae of cabbage leaf webber and diamond back moth and found that the standard controls; Dipel and HD1, had the highest mortality rates (90.00 and 80.00% against leaf webber, respectively), compared to the native isolates 2422c (80.00%). Similarly, for Plutella xylostella, the mortality rates with the reference strains were 99.97 and 86.67 per cent which can comparable to native strain 2458c with 80.00 per cent mortality.
Similarly, Lalitha and Muralikrishna (2012) reported a mortality range of 10 to 93.33 per cent among the 114 native B.t isolates against first and third instar S. litura larvae. While the highest mortality was recorded in the
Table 1. Mean per cent mortality of third instar larvae of S. frugiperda at different concentrations of native
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
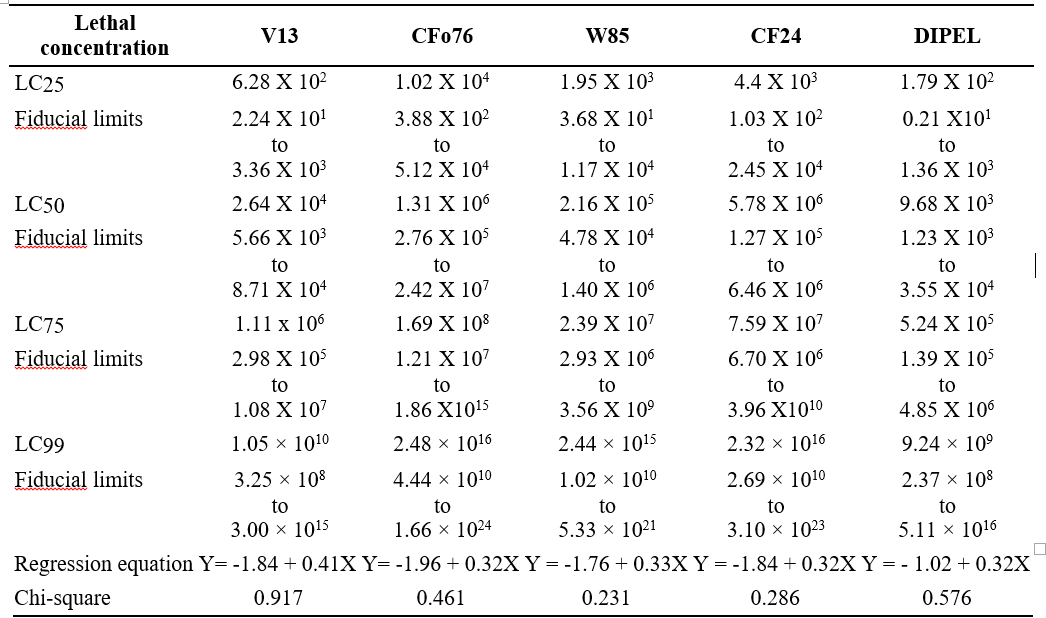
Table 2. Lethal concentration (LC25, LC50, LC75 and LC99) values for native B.t isolates against third instar larvae of S. frugiperda
reference strain, HD1(93.33 and 76.67 per cent in first and third instar larvae, respectively).
Thilagavathi et al. (2020) reported the toxicity of four B.t. isolates against third instar larvae of P. xylostella and the isolate CC recorded the maximum mortality of 95.33 per cent comparable to the standard check HD1 98.31 per cent.
Determination of Lethal Concentration (LC) of B.t
isolates against S. frugiperda (Table 2)
For all the four native isolates of B.t tested; LC25 values ranged from 6.28 × 102 to 1.02 × 104 CFU/ml. The lowest LC25 value was recorded in V13 (6.28 × 102 CFU/ml) followed by W85 with 1.95 × 103 CFU/ml. whereas the highest value was recorded in the isolate CFo 76 with 1.02 × 104CFU/ml
The LC50 values for the four native isolates tested were in the range from 2.64 × 104 to 5.78 × 106 CFU/ml. The lowest LC50 was recorded in V13 isolate (2.64 × 104 CFU/ml) followed by W85 isolate (2.16 × 105CFU/ml), while the highest LC50value was noted in the isolate CF24.
The LC75 for the four native isolates tested ranged from 1.11 × 106 to 1.69 × 108 CFU/ml, with the lowest LC75 in V13 (1.11 × 106CFU/ml) followed by W85 2.39× 107 CFU/ml. The highest LC75 value was recorded in the isolate CF24 (7.59 × 107CFU/ml).
LC99 values of the four native isolates ranged from 1.05 × 1010 to 2.48 × 1016 CFU/ml. The lowest LC99 value was observed in V13 (1.05 × 1010CFU/ml.) followed by W85 with 2.44 × 1015CFU/ml. Meanwhile the highest LC99 value was recorded in CFo76 with 2.48 × 1016 CFU/ ml while for the CF24 was 2.32 × 1016CFU/ml. which was similar to the CFo76 isolate. Similar to the per cent larval mortality from earlier bioassay results (Table 1) the lowest LC25, LC50, LC75 and LC99 was observed in commercial Dipel (Table 2) indicating its highest efficacy.
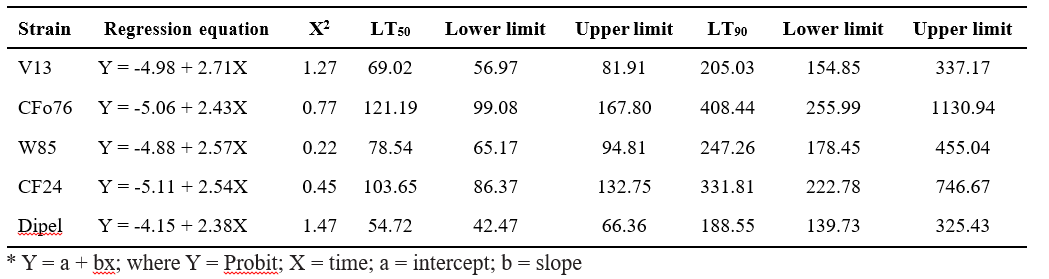
Table 3. Lethal time (LT) values for native B.t isolates against third instar larvae of S. frugiperda
However, among the native isolates tested the lowest LC25, LC50, LC75 and LC99was observed in the isolate V13 ranking it as most effective isolate. Valicente and Lana (2008) conducted bioassay studies of S. frugiperda with B. t and determined the LC50 by using doses ranging from 103 to 109 spores /ml against two-day old healthy fall army worm larvae. LC50 was 8.21 × 106 spores /ml for strain 344 while strain 1644 showed LC50 of 2.07 × 106 spores /ml.
Murali Krishna et al. (2018) recorded the least lethal concentration in HD1 strain of B.t. as 9.56 × 10 4 CFU/ml followed by 9.76 × 104 CFU/ml with F493 isolate and
1.90 × 105 CFU/ml with N30 isolate when screened against third instar larvae of S. litura.
Determination of Lethal Time (LT) of B.t isolates against S. frugiperda
Among the four native isolates tested, the least LT50 value was recorded in the V13 isolate with 69.02 hours followed by W85 with 78.54 hours. Whereas for the commercial check, Dipel the LT50 value was 54.72 hours. Regression equation, fiducial limits and chi square values were presented in Table 3.
Prabagaran et al. (2002) reported that LT values of live B.t. strains, viz., PBT-782, PBT 372, PBT-574, PBT801 and PBT-716 were 25.46, 36.81, 48.18, 50.35 and 73.53 h, respectively.
Similar to the present investigation, Murali Krishna et al. (2018), reported that four isolates (E468, E493, N30, N115) among 21 B.t. isolates were found to be potent isolates with a median lethal time of 74.28 h 78.52 h, 88.68 h, and 95.70 h, respectively.
Among the four native isolates tested, V13 isolate showed the highest per cent larval mortality (86.66%),
Lowest values of LC50 (6.28 × 102 CFU/ml) and LT50 (69.02 h) indicating that the isolate V13 could be the most potential B.t native isolate for the management S. frugiperda.
LITERATURE CITED
Ali, K., Wakil, W., Zia, K and Sahi, S.T. 2015. Control of Eariasvittella (Lepidoptera: Noctuidae) by Beauveria bassiana along with Bacillus thuringiensis. International Journal of Agriculture & Biology. 17: 773 778.
Aneja, K.R. 2008. Experiments in Microbiology, Plant Pathology and Biotechnology. New Age International (P) Limited Publishers.
- 1999. Insecticidal Activity of Culture Supernatants from Bacillusthuringiensis Berliner Strains against Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) Larvae. Anais da Socieda de Entomologica do Brasil. 28 (4): 675-685.
Figueiredo, M. L. C., Penteado Dias, A. M and Cruz, I. 2015. Danos provocados por Spodoptera frugiperda na produ~llo de matc!rla seca e nos rendimentos de graos, na cultura do milho. (Comunicado Tecnico, 130). Embrapa 1 CNPMS, Sete Lagoas, Brazil. 6.
Lalitha, C and Muralikrishna, T. 2012. Laboratory evaluation of native Bacillus thuringiensis isolates against Spodopteralitura (Fabricius). Current Biotica. 5 (4): 428-435.
Lima, M. S., Silva, P. S. L., Oliveira, O. F., Silva, K. M.
- and Freitas, F. C. L. 2010. Corn yield response to weed and fall armyworm controls. Planta Daninha, 28: 103-111.
Montezano, D.G., Specht, A., Sosa-Gómez, D.R., Roque- Specht, V.F., Sousa-Silva, J.C., Paula-Moraes, S.V., Peterson, J.A and Hunt, T.E. 2018. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. African Entomology. 26(2): 286-300.
Muhammad, N., Juma. I.M and Hongxia, H. 2016. Current status and advancement of biopesticides: Microbial and botanical pesticides. Journal of Entomology and Zoology Studies. 4 (2): 241-246.
Muralikrishna, T., Devaki, K., Swarna, B., Abhijit, N and Naresh, M. 2018. Computation of LC50 against Spodoptera litura (Fab.) for Bacillus thuringiensis isolates from native soil samples of Andhra Pradesh. Journal of Pharmacognosy and Phytochemistry. 7(4): 174-178.
Nethravathi, C.J and Hugar, P. S. 2010. Bioefficacy of Chikkamagalur Native Bacillus thuringiensis isolates against Lepidopteran Insects. Journal of Biological Control. 24(3): 282-284.
Pavani, S. 2019. Isolation and characterization of native isolates of Bacillus thuringiensis (Berliner) from cotton cultivated soils of Guntur district, AP. M.Sc. (Ag) thesis. Acharya N G Ranga Agricultural University, Guntur.
Prabagaran, S.R., Nimal, S.J and Jayachandran, S. 2002. Phenotypic and genetic diversity of Bacillus thuringiensis strains isolated in India active against Spodoptera litura.Applied Biochemistry and Biotechonology. 102: 213-226.
Sabbour, M.M. 2003. Combined effects of some microbial control agents mixed with botanical extracts on some stored product insects. Pakistan Journal of Biological Sciences. 6(1): 51-56.
Sharanabasappa, Kalleshwaraswamy, C.M., Asokan, R., Mahadeva, H.M.S, Maruthi, M.S and Pavithra, H.B.018 First report of the fall Armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae) an Alien invasive pest on Maize in India. Pest management in Horticultural Ecosystems. 24: 23-29.
Taggar G. K., Ravinder, S., Veena, K and Harpreet K.C. 2014. Preliminary Evaluation of Native Bacillus thuringiensis Isolate and Microbial Formulations against Pod Borer, Helicoverpa armigera in pegion pea. Journal of Pure and Applied Microbiology. 8(3): 2491-2495.
Thilagavathi, S.S., Prasad, G and Ramalakshmi, A. 2020. Bio Efficacy of Bacillus thuringiensis isolates against Diamond Back Moth (Plutella xylostella L.) on Cauliflower Plant in Tamil Nadu, India. International Journal of Plant and Soil Science. 32(1):10-20.
Togbe, C. E., Zannou, E., Gbehounou, Z., Kossou, D and Arnold, V.H. 2014. Field evaluation of the synergistic effects of neem oil with Beauveria bassiana and Bacillus thuringiensis var. Kurstaki. International Journal of Tropical Insect Science. 34(4): 248-259.
Valicente, F.H and Lana, U.G.D.P. 2008. Molecular characterization of the Bacillus thuringiensis (Berliner) strains 344 and 1644, efficient against fall armyworm Spodoptera frugiperda (J.E. Smith). Brazilian Magazine of Corn and Maize. 7(3): 195- 209.
- Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
- Brand Preference of Farmers for Maize Seed
- Issues That Consumer Experience Towards Online Food Delivery (Ofd) Services in Tirupati City
- Influence of High Density Planting on Yield Parameters of Super Early and Mid Early Varieties of Redgram (Cajanus Cajan (L.) Millsp.)
- Influence of Iron, Zinc and Supplemental N P K on Yield and Yield Attributes of Dry Direct Sown Rice
- Effect of Soil and Foliar Application of Nutrients on the Performance of Bold Seeded Groundnut (Arachis Hypogaea L.)

