Effect of Different Chemicals and Biocontrol Agents On Growth of Rhizoctonia Solani Incitant Banded Leaf and Sheath Blight on Barnyard Millet Under in Vitro Conditions
0 Views
T . PALLAVI*, T.S.S.K. PATRO, K.B. PALANNA, M. GURIVI REDDY AND M. SHANTHI PRIYA
Department of Plant Pathology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
An in vitro experiment was conducted to evaluate the efficacy of different chemicals viz., tebuconazole + trifloxystrobin
75WG @ 0.05%, propiconazole 25 EC @ 0.1% and biocontrol agents viz., Trichoderma asperellum, Pseudomonas fluorescens, Bacillus subtilis on Rhizoctonia solani that causes banded leaf and sheath blight on barnyard millet. The chemicals were evaluated at the recommended dose, 50% of recommended dose, 25% of recommended dose and the results revealed that the growth of the pathogen was inhibited completely i.e., 100% and among the biocontrol agents Trichoderma asperellum was found to be more effective than Pseudomonas fluorescens, Bacillus subtilis. Trichoderma asperellum inhibited the growth of the pathogen upto 71.56%, while Bacillus subtilis and Pseudomonas fluorescens inhibited the growth of the pathogen upto 58.59% and 54.08% respectively.
KEYWORDS: Barnyard millet, banded leaf and sheath blight, chemicals, biocontrol agents.
INTRODUCTION
Barnyard millet (Echinochloa frumentacea) belongs to the family Poaceae, and the sub-family Panicoideae (Clayton and Renvoize, 2006). It is a short duration crop among all the millets with approximately six weeks crop growing period. It is known by various vernacular names like Shyama, Sanwa, Oodalu, Khira, Kutdrivalli all over India (ICRISAT, 2022). It is widely grown in India, China, Japan, Pakistan, Africa and Nepal (Paschapur et al., 2021) and offers food security to many people of Asian and African countries, especially high altitude and tribal regions. Andhra Pradesh has 0.022 M ha area under millet cultivation with a production of 22 MT and a productivity of 1000 kg ha-1 (Indiastat, 2019-20). It is highly nutritive and has the lowest glycemic index (42.3%) which makes it an ideal food for the people suffering from lifestyle diseases like diabetes mellitus and cardiovascular problems (Anitha et al., 2021). It tolerates many extreme environmental conditions and is one of the best remunerative crops to small and marginal farmers. However, it is prone to several biotic stresses of which fungal diseases like grain smut (Ustilago panici- frumentacei), leaf blast (Pyricularia grisea) and banded leaf and sheath blight (Rhizoctonia solani) results in considerable yield losses in barnyard millet. A versatile range of diseases are caused by the banded leaf and sheath blight (BLSB) pathogen which includes damping off, seed decay, aerial blight, stem canker, seed decay and root rot. Yield loss assessment studies revealed that the potential yield loss caused by the pathogen ranged from 52.7 to 67.2 per cent (Palanna et al., 2021). In this context, different chemicals and biocontrol agents were evaluated to know their efficacy in inhibiting the growth of the pathogen under in vitro conditions.
MATERIAL AND METHODS
Different biocontrol agents viz., Trichoderma asperellum, Pseudomonas fluorescens, Bacillus subtilis were evaluated for their efficacy to inhibit the growth by dual-culture technique and the chemicals, tebuconazole + trifloxystrobin 75 WG, propiconazole 25 EC were evaluated at recommended dose, 50% of recommended dose, 25% of recommended dose by poisoned food technique. Tebuconazole + trifloxystrobin 75 WG was evaluated at 0.05%, 0.025%, 0.0125% while, propiconazole 25 EC was evaluated at 0.1%, 0.5%, 0.025%. The biocontrol agents were collected from Department of Biological Control, Vizianagaram. Chemicals were evaluated by poisoned food technique and to 50 ml of sterilized distilled water, required quantity of double strength fungicide was added and mixed thoroughly. This solution was poured into 50 ml of sterilized cool molten double strength PDA medium, mixed thoroughly and poured into Petri plates. Five mm actively growing fungal disc from 4 days old culture was inoculated at the centre and then incubated at 28±1oC. Four replications were maintained for each fungicide. Medium without fungicide was kept as control. Biocontrol agents were tested by dual-culture technique and to test the efficacy of antagonistic fungus, 20 ml of sterilized melted PDA was plated in Petri plates (9 cm) and allowed to solidify. Mycelial discs measuring six mm diameter from three-day old cultures of both fungal antagonist and the test pathogen were placed at equidistant on sterile Petri plate containing PDA medium. The Petri plates with pathogen inoculated at one end alone, served as control. The Petri plates were then incubated at 28 ± 2°C. Four replications were maintained in each treatment. Growth of Trichoderma, test pathogen and zone of inhibition were measured after recording full growth of the pathogen in control plate. Per cent inhibition of mycelial growth of test pathogen in dual-culture and poisoned food technique was calculated by the formula:
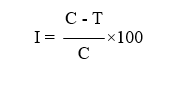
where,
I = Per cent inhibition in growth of test pathogen
C = Radial growth of test fungus (mm) in control
T = Radial growth of test fungus (mm) in treatment
RESULTS AND DISCUSSION
Biocontrol agents viz., Trichoderma asperellum, Bacillus subtilis and Pseudomonas fluorescens and the chemicals like propiconazole 25% EC, tebuconazole
+ trifloxystrobin 75 WG were evaluated in vitro at the recommended dose i.e., 0.1% for propiconazole and 0.05% for tebuconazole + trifloxystrobin and also at 50%, 25% of the recommended dose.
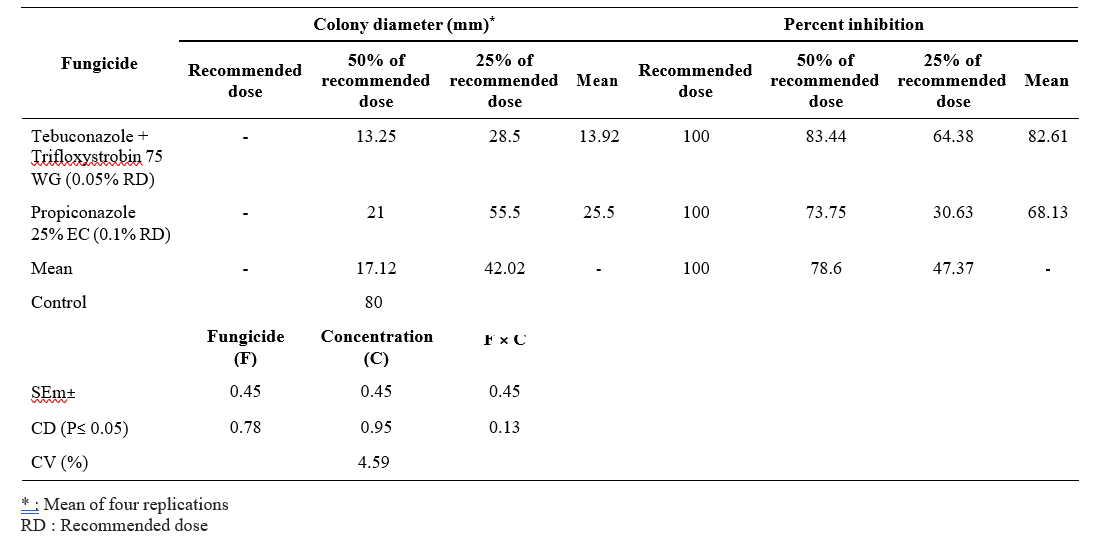
The growth of the pathogen was inhibited at different levels of which tebuconazole + trifloxystrobin @ 0.05%, propiconazole @ 0.1% was found to be effective when compared to tebuconazole + trifloxystrobin @ 0.025%, 0.0125% concentration and propiconazole @ 0.05%, 0.025% concentration. The radial growth of pathogen when tested with tebuconazole + trifloxystrobin @ 0.05%, 0.025%, 0.0125% was 0, 13.25 mm, 28.5 mm respectively, while the inhibition percent over control was 100%, 83.44%, 64.28% at 0.05%, 0.025%, 0.0125% respectively. In case of propiconazole 25 EC @ 0.1%, 0.05%, 0.025%, the radial growth of the pathogen was 0, 21 mm, 55.55 mm and the percent inhibition over control was 100%, 73.75%, 30.63% respectively (Table 1).
Among the biocontrol agents, Trichoderma asperellum was found to be effective among all as it inhibits the growth of R. solani upto 72.19%, while Bacillus subtilis inhibits the growth by 59.22%. However, Pseudomonas fluorescens was capable of inhibiting the pathogen by 54.22% only. The results revealed that the fungicides were effective in inhibiting the complete growth of the pathogen only at the recommended dose even under lab conditions (Table 2, Plate 1)..
The results corroborated with the earlier findings of Usendi et al. (2020) who reported that Trichoderma asperellum (71.38% inhibition) was more effective in inhibiting the growth of the pathogen among different biocontrol agents tested which includes Pseudomonas fluorescens and Bacillus subtilis. In contrast to the present results, it was also reported that Pseudomonas fluorescens (64.44%) was more effective than Bacillus subtilis (61.38%). Among the chemicals tested, it was reported that propiconazole 25% EC @ 0.1% and tebuconazole + trifloxystrobin 75WG @ 0.1% inhibited the growth of the pathogen upto 100%.
The results were also in accordance with the findings of Kumar et al. (2017) who reported that propiconazole and carbendazim @ 1000 ppm could completely inhibit mycelial growth of R. solani. Similarly, Mahantesh et al. (2018) tested efficacy of propiconazole (5 to 100 ppm) on rice sheath blight pathogen R. solani under both in vitro and in vivo conditions and reported 100% mycelial inhibition was observed with propiconazole from 25 ppm onwards and under field conditions low disease severity of 45.76% was reported with 41.37% increase over control with yield of 67.72 q ha-1.
In conclusion, the present investigation revealed that the chemicals were more effective than biocontrol agents in inhibiting the growth of the pathogen and the chemicals were effective at the recommended dose i.e., tebuconazole + trifloxystrobin 75 WG @ 0.05% and propiconazole 25 EC @ 0.1%, while among the biocontrol agents Trichoderma asperellum was found to be more effective than Bacillus subtilis followed by Pseudomonas fluorescens.
Table 1. In vitro efficacy of fungicides against R. solani

LITERATURE CITED
Anitha, S., Kane-Potaka, J., Tsusaka, T.W., Botha, R., Rajendran, A., Givens, D.I., Parasannanavar, D.J., Subramaniam, K., Prasad, K.D.V., Vetriventhan, M and Bhandari, R.K. 2021. A systematic review and meta-analysis of the potential of millets for managing and reducing the risk of developing diabetes mellitus. Frontiers in nutrition. 386.
Clayton, W.D and Renvoize, S.A. 1986. Genera graminum. Grasses of the world. Genera Graminum. Grasses of the World. 13.
ICRISAT, 2022. http://genebank.icrisat.org/IND/ Dashboard Crop/Barnyard millet.
Indiastat, 2022. https://www.indiastat.com/data/ agriculture/small-millets.
Kumar, V., Chaudhary, V.P., Kumar, D., Kumar, A, Sagar, S and Chaudhary, S. 2017. Efficacy of botanicals and fungicides against Rhizoctonia solani inciting sheath blight disease on Rice (Oryza sativa L.). Journal of Applied and Natural Science. 9(4): 1916 -1920.
Mahantesh, Singh, O., Vishwanath and Singh, D. 2018. Efficacy of fungicides for the management of Sheath Blight of rice. Chemical Science Review and Letters. 7(27): 714-718.
Palanna, K. B, T. S. S.K. Patro, I.K. Das, S. Saralamma, H. Raveendra Contents Laxmi Rawat, Savita Ekka and M,Rajesh, Prahlad Netam, H. Rajashekara, and A.K.Jain. 2021. Plant Pathology Annual Progress Report: AICRP Small Millets Kharif 2020-21. 18- 19.
Paschapur, A.U., Joshi, D., Mishra, K.K., Kant, L., Kumar, V. and Kumar, A. 2021. Millets for Life: A Brief Introduction. Springer, Singapore. 1-32.
Usendi, P.N., Giri, G.K. and Kabade, S.H. 2020. Evaluation of Fungicides, Bio-agents and Botanicals against Rhizoctonia spp. incitant of sheath blight of rice. International Journal of Current Microbiology and Applied Sciences. 9(8): 3039-3046
- Genetic Divergence Studies for Yield and Its Component Traits in Groundnut (Arachis Hypogaea L.)
- Correlation and Path Coefficient Analysis Among Early Clones Of Sugarcane (Saccharum Spp.)
- Character Association and Path Coefficient Analysis in Tomato (Solanum Lycopersicum L.)
- Survey on the Incidence of Sesame Leafhopper and Phyllody in Major Growing Districts of Southern Zone of Andhra Pradesh, India
- Effect of Organic Manures, Chemical and Biofertilizers on Potassium Use Efficiency in Groundnut
- A Study on Growth Pattern of Red Chilli in India and Andhra Pradesh