Development and Validation of Gc-Ecd Method for Determination of Profenophos in Pigeonpea
0 Views
K. SUNEETHAMMA, K. MANJULA, K. DEVAKI, M. PRADEEP AND P. LAVANYA KUMARI
Department of Entomology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
A modified quick, easy, cheap, efficient, rugged, and safe (QuEChERS) method coupled to Gas Chromatography with Electron Capture Detection was developed for the determination of profenophos residues in pigeonpea standing crop. Extraction and cleanup parameters were optimized; thus, the original QuEChERS method was modified to minimize solvent usage. After optimization, the method was validated by evaluating the analytical curves, linearity, Limits of Detection (LOD) and Limits of Quantification (LOQ) and accuracy (recovery). Retention time was 22.018 minutes and minimum detectable and quantifiable limits were 0.05 and 0.15 µg mL-1, respectively. Good linearity (R2=0.9935) of the calibration curves was obtained over the range from 0.1 to 1 µg mL-1. Good recovery in pigeonpea flower matrix was obtained with 90 to 98 per cent at 0.05 to 0.5 ppm with RSD value < 20% (0.93%). Results indicated that the developed method is rapid and easy to perform and making it applicable for analysis of profenophos in pigeonpea matrix. At 0 (2 hours) day after the insecticidal application an initial deposit of 0.72 mg kg-1 was recorded. The residues reached BDL on 7th day, exhibiting cent per cent dissipation with the half-life of 2.4 days.
KEYWORDS: Profenophos, pigeonpea, GC-ECD, linearity, recovery, LOD, LOQ, RSD.
INTRODUCTION
Pigeonpea (Cajanus cajan L.) commonly known as red gram or tur. It is an important pulse or grain legume crop grown in semiarid and subtropical regions of the world (Chandrakala et al., 2022) and major pulse crop among the pulses grown in India. It is the world’s fifth-most important pulse crop (Pratibha et al., 2015). Pigeonpeas crop is being attacked by more than 250 species of insects which belonging to 8 different orders and 61 different families (Lal and Katti, 1997). The major pests are gram pod borers whose infestation is in synchrony with flower initiation to pod maturity. The pigeonpea pod borer complex comprises of gram pod borer, Helicoverpa armigera Hubner; plume moth, Exelastis atmosa Walshingham, spotted pod borer, Maruca vitrata and pod fly, Melanagromyza obtusa Malloch etc. H. armigera alone contributes loss up to 50 per cent (Dodia et al., 2009). Hence, to reduce the substantial yield loss in pigeonpea, it is inevitable to manage these wide ranges of pest group effectively.
Profenophos is an organophosphorus insecticide, chemically called as O-4-bromo-2-chlorophenyl O-ethyl Spropyl phosphorothioate (IUPAC) and O-(4-bromo- 2-chlorophenyl) O-ethyl Spropyl phosphorothioate (CAS). It is an extremely toxic and persistent chemical as per the toxicity classification. It is a non-systemic and acaricide with contact and stomach action and being extensively used for the control of lepidopteran group larvae, whitefly and mites on cotton, pigeonpea, chilli and vegetable crops (Sharma et al., 2018). Further, profenophos efficacy on flower borer and flower fly infesting pigeonpea was in the range of 25 to 85% when used as ovicide and larvicide (Srivastava and Mohapatra, 2003). The European Union limits (Maximum Residue Limitss) for profenophos in pigeonpea is 0.01 mgkg-1. It was reported that, the initial deposits of profenophos was much higher in the tomato than the cypermethrin (Gupta et al., 2011) indicate its high persistence nature. Several reviews have examined persistence and dissipation of profenophos and residue analysis in fresh and edible crops such as tea leaves (Pramanik et al., 2005), okra (Paras et al., 2005), green and cured cardamom (Renuka et al., 2006), chillies (Reddy et al., 2007), brinjal (Nigam et al., 2009; Mukharjee et al., 2012. As most of the laboratories are not equipped with highly sensitive chromatography instruments like gas/ liquid chromatography tandem mass spectrometry (GC- ms/ms and LC- ms/ms) for routine analysis and there is very scarce literature on analysis of profenophos in pigeonpea by GC-ECD, therefore, a sensitive analytical gas chromatographic method capable of estimating in microquantities is required. The objective of this study is to improve the extraction procedure and develop simple, sensitive method which can be conveniently used for the detection and determination method of profenophos.
MATERIAL AND METHODS
Instrument & Equipment
The following instrument and equipment were used for the study to carry out the sample extraction in the Pesticide Testing Laboratory, Institute of Frontier Technology, Tirupati. They were Balance (upto 0.02 mg and capacity- 6.0 kg), Blender MG-198 W. Homogenizer to pulverize, high volume centrifuge, Vertex, low volume centrifuge, Evaporator (Rapid Mini EC System, Crescent Scientific Company), Rapid mini EC system and Nitrogen air generator, Gas Chromatography- Electron Capture Detector (GC-ECD) were used to develop and validate a method in pigoenpea matrix.
Apparatus and Reagents
Centrifuge tubes (15 and 50 mL), Measuring cylinders, Micropipettes, Vials, Acetonitrile (HPLC analytical grade), Water (HPLC grade), Acetonitrile (HPLC grade), n-Hexane, Methanol (HPLC grade), Ethyl Acetate (HPLC grade), Saturated sodium chloride, Sodium Sulphate, Magnesium Sulphate (MgSO4) anhydrous, Primary Secondary Amine (PSA) and Graphitised Carbon Black (GCB) were utilized for the sample extraction in the present study.
Preparation of standard solutions
Profenophos standard stock solution (1000 µg mL-1) was prepared by weighing 10 mg (± 0.1) of certified reference material in a calibrated 10 mL volumetric flask and volume made up with solvent n-hexane. An intermediate standard solution of 100 µg mL-1 was prepared by pipetting out 1 mL of stock solution in to 10 mL volumetric flask and volume made up using n-hexane. A working standard of 1 µg mL-1 was prepared in n-hexane and further the calibration standard solution ranging from 0.05 to 1 µg mL-1 were prepared. The matrix match standards at the similar concentrations were prepared by using the control pigeonpea flower samples extract obtained through sample preparation by adding the rewuired volume of analyte.
Chromatographic Conditions
Gas Chromatography (GC Schimadzu 2010) was used to analyze profenophos using a column EB-5 (30m x 0.25mm x 0.25µm) coated with 1% phenyl- methylpolysiloxane (0.25 μm film thickness) and a 63Ni electron-capture detector (ECD). General operational conditions were as follows: The column temperature program was set to 500 C for 1 minute, then increased to 1200C at 200C/min, held for 0 minute and raised to 2800C at 500C/min for 13 minutes. The total programming time was 40 minutes, with an injection volume of 10 μL/min and a nitrogen flow rate of 0.79 mL/min. The carrier gas used was 99.99 percent pure N2. For the elution of the seven test chemicals, injector port temperatures were maintained at 2600C and detector temperatures at 2800C. The analyte in the samples was identified by comparing the retention time of the matching matrix-matched calibration standard and quantification was done using external calibration curves created with a four-point matrix-matched calibration standard.
Feld Experiment
A filed experiment was conducted to study the dissipation pattern of profenophos in pigeonpea flowers at Dryland farm, S.V. Agricultural College, Tirupati during rabi, 2022-23. Profenophos spraying was done during 50 % flowering stage and the pigeonpea flower samples were collected on intervals of 1,3,5,7,10,15,20 and at harvest to study the dissipation patterns in pigeonpea flowers. Laboratory analysis was carried out at Pesticide residue testing Laboratory, Institute of Frontier Technoliogy, RARS, Tirupati.
Method Validation
The method was validated by evaluating analytical curves and linearity, limit of detection (LOD), limit of quantification (LOQ) and accuracy (recovery)
a) Analytical Curve and Linearity
The linearity of the instrument and the method was evaluated by analytical curves with the concentration levels from the LOQ of compound, that is, 0.05 to 1.0 mg/ mL with three replicate injections per concentration.
b) Limit of Detection and Quantification
The sensitivity of the method was determined using ratio between the estimated standard deviation of the linear coefficient and the slope of the analytical curve. The LOD and LOQ for profenophos were determined.
c) Recovery
The pigeonpea flower samples were collected from untreated control plots was brought to the laboratory and were homogenized using high volume homogenizer and each homogenized samples of 10 g was transferred to 50 mL centrifuge tubes. Extraction and clean up was done by QuEChERS method. The required quantity of intermediary standard prepared from CRM of respective standards were added to homogenized pigeonpea flower samples to get required fortification levels and each replicated thrice. These fortification levels were selected to know the suitability of the method to detect and quantify insecticides in pigeonpea samples below MRLs of Codex Alimentarius Commission (CAC). Then 10 mL of HPLC ice cold water and 15 mL of acetonitrile was added and homogenized the sample at 14000-15000 rpm for 3 minutes. To this, 6 g of sodium sulphate and 1.5 g of sodium acetate was added and centrifuged for 3 minutes at 4500 rpm to separate the organic layer. Then 12 mL of aliquot was transferred into 15 mL centrifuge tube containing 0.60 g magnesium sulfate and 0.2 g PSA (also add 10 mg of Graphitised Carbon Black to remove carotenoid content), vortexed for 30 seconds and centrifuged for 3 minutes at 2500- 300 rpm. A supernatant layer of 1 mL was taken into 15 mL tube for evaporation using rapid mini EC system (low volume concentrator using gentle stream of nitrogen at 350 C) and reconstituted with 2 mL n-hexane for analysis with
d) Calculations Residues (mg/kg)
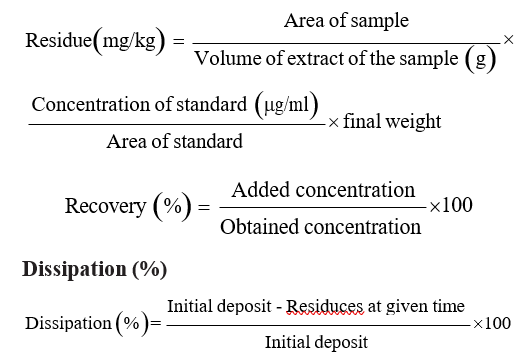
Half-life (RL50)
RL50 is the time in days required to reduce the insecticide residues to half of its initial deposits.
Mathematically, it is
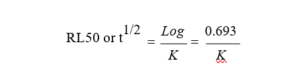
Where K is the dissipation rate constant
Prediction of approximate time required to dissipate the residue below maximum residue limit
The period allowed to except the residues to reach below the tolerance limit after treatment for use of the treated material will be calculated by using the formula (Blinn and Gunther, 1955).
Y = a + bX where, Y = log of tolerance limit
a = log of initial deposit
b = slope of the regression line X = intercept
Equation of first-order kinetics
Ct = C° x ekt
The residue was calculated with the first-order equation
where, Ct is the concentration (mg/kg),
Co is the initial concentration (mg/kg),
k is the dissipation rate constant,
t is time (days) after application
RESULTS AND DISCUSSION
Under the optimised GC-ECD conditions, the retention time for profenophos was 22.018 minutes. N-hexane blank did not contain any residue.
Method validation for estimation of profenophos in pigeonpea flowers
The analysis of profenophos residues in pigeonpea flowers was validated by calculating and assessing
various performance parameters such as linearity, LOD (Limit od Detection), LOQ (Limit of Quantification) and recovery etc., as per the SANTE/11813/2017 guidelines. The pigeonpea crop grown without application of insecticides was used for the spiking to establish LOD and LOQ method. The LOQ of the method for profenophos was 0.15 and LOD was 0.05 µg ml-1. The mean recovery of profenophos from pigeonpea flower was within the acceptable range 70 to 120 per cent when spiked at 0.05
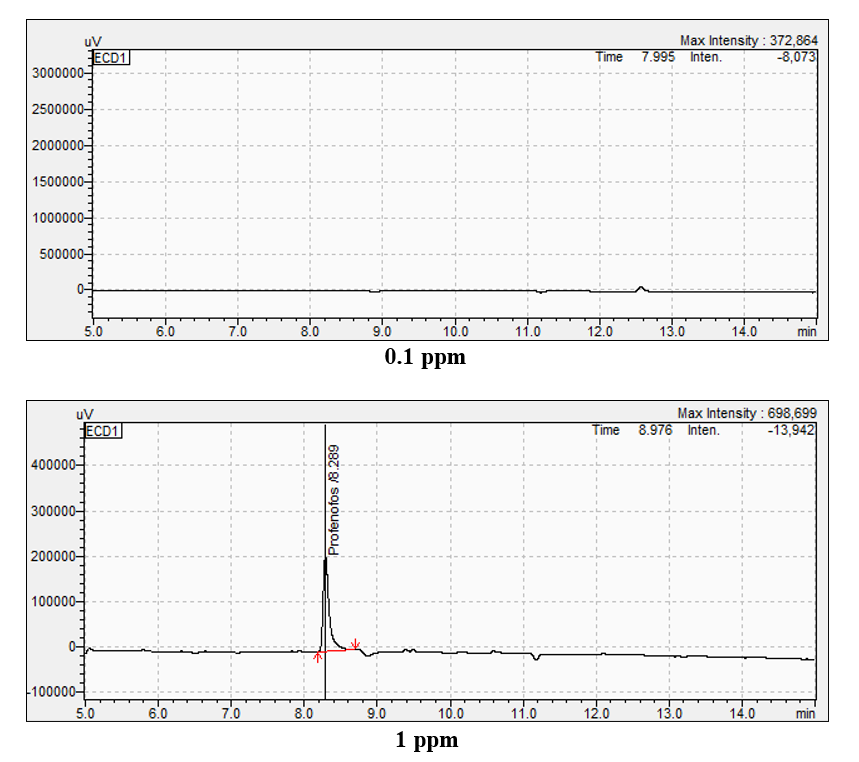
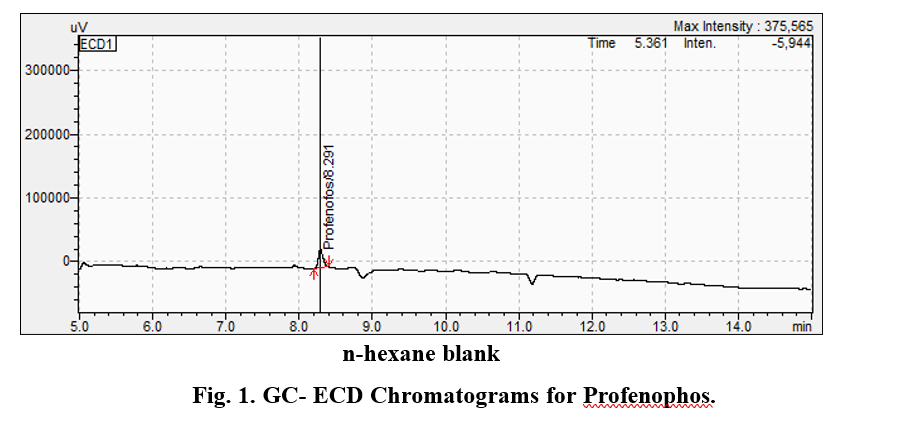
Table 1. Details of GC-ECD operational parameters of profenophos
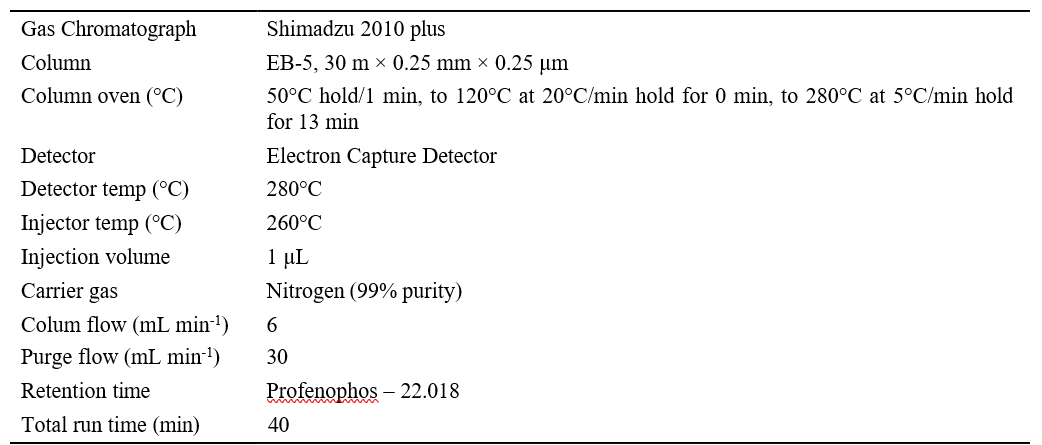
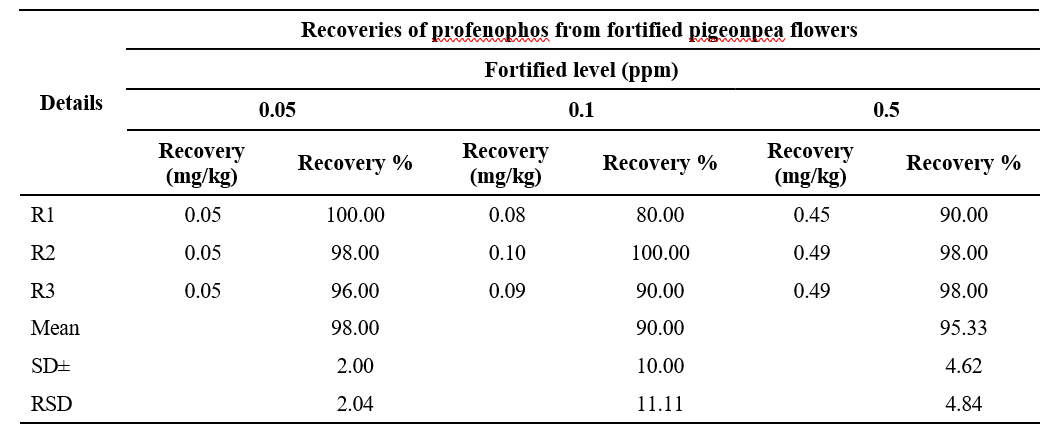
Table 2. Recoveries of profenophos from fortified pigeonpea flowers
|
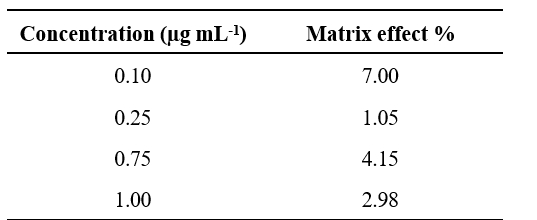
to 0.50 µg ml-1. The recoveries of profenophos were in the range of 90 to 98 per cent for pigeonpea flowers. The precision in terms of repeatability was associated with recovery % and by calculating Relative Standard Deviation (RSD %) (Table 2). The RSD ranged from 12.29 to 20.06 per cent for profenophos with pigeonpea matrices. Linearity was achieved by four different concentrations (0.1 to 1.00 µg/ml) with calibration correspond to R2=0.9935 as shown in Fig. 2 in flowers. Matrix effect was calculated from the data and was observed to be within the prescribed limits shown in Table 3.
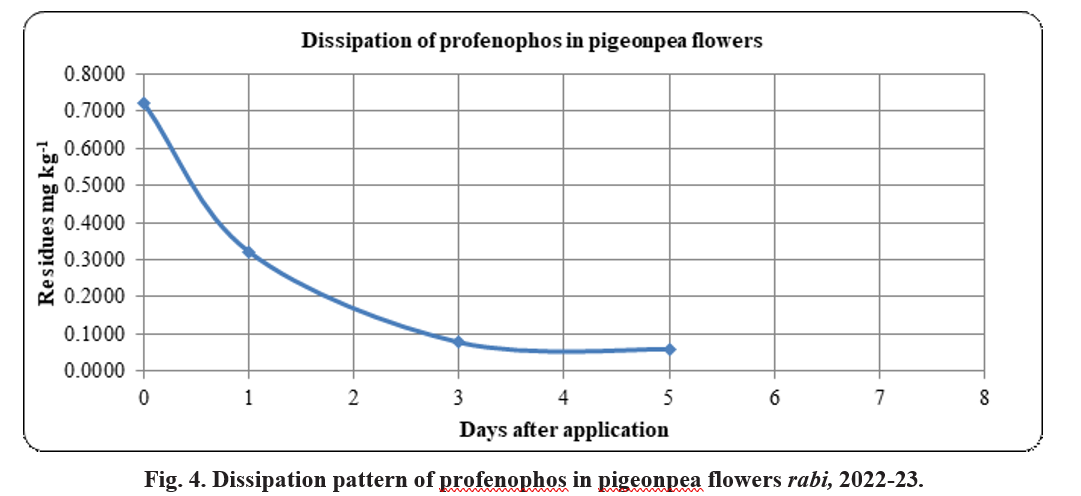
Dissipation pattern of profenophos in pigeonpea flowers
At 0 (2 hours) day after the insecticidal application an initial deposit of 0.72 mg kg-1 was recorded and decreased to 0.32 mg kg-1 in flowers at 1 day after application. The same was dissipated to 89.14 per cent at 3 days after application showing residual amount of 0.08 mg kg-1. The residual amount of 0.06 mg kg-1 was observed at 5th day. The residues reached BDL on 7th day, exhibiting cent per cent dissipation (Table 4). The half- life of 2.4 days was observed. The regression equation was with y = -0.1695x + 0.7768 with R² = 0.8408 and
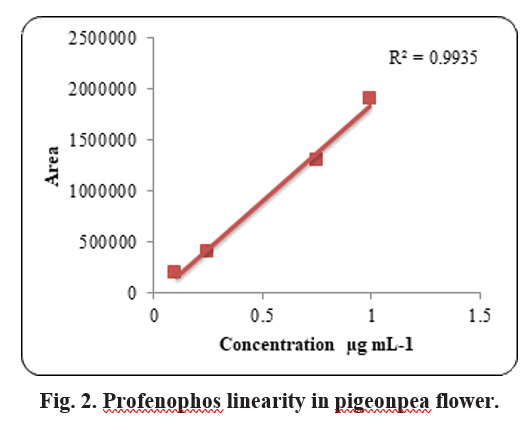
Table 3. Matrix effect (%) of profenophos in pigeonpea flowers
the dissipation followed the first order kinetics Ct=0.827 e-0.5894x and R² = 0.9337 (Fig 3). The MRL values are not available for profenophos in pigeonpea flowers by either CAC or by FSSAI, hence the day at which the residues reached BDL (7 days) was considered as safe waiting period.
The present investigated results were in line with the recordings on dissipation pattern noticed in pigeonpea wherein profenophos dissipated quickly between 0-1 day in both the doses (Naik et al., 2020). Mukherjee et al. (2012) who reported the initial deposits (2 hr after application) of profenophos on brinjal fruits were 0.575 and mg kg-1 for recommended. The residues dissipated with time and on the 7th day, residues were below determination limit at the recommended rate of application. Brar (2013) observed dissipation of profenophos on brinjal fruits and found the initial deposits of 1.966 mg kg-1 with half-life value of 1.5 days. In okra recorded the half-life of 1.35 days after the spray followed a biphasic dissipation pattern with faster dissipation in phase I (0-1 days) and manifesting slower rate of dissipation in phase II (1-15 days) as reported by Paras et al. (2005).
Table 4. Data on dissipation of profenophos in pigeonpea flowers during rabi, 2022-23

Shashi et al. (2014) also reported the calculated half- life of profenophos in brinjal is 1.57 days. Gupta et al. (2011) reported that residues of profenophos dissipated with half-lifes of 2.2- 5.4 days and Sahoo et al. (2004) reported that profenophos spray on brinjal at 50% flowering stage and subsequently at 15 days intervals, resulted in to initial deposit of 1.37 mg kg-1 dissipating to BDL in 15 days. However, the studies conducted by various workers on dissipation on profenophos on different crops clearly indicate that when applied at recommended dose, the initial deposits are less than 3 mg kg-1 and dissipate to BDL in 7-10 days depending on the crop.
The proposed method showed acceptable repeatability and provides an alternative route to determine the profenophos compounds from flowers with a safer way with no compromise in sensitivity. Validation of the method has been shown with parameters of linearity, precision, LOD and LOQ, accuracy and specificity. The method gives more efficiency, reduced amount of chemicals and more sensitivity in comparison to earlier reported methods. The method can be applied to determine pesticide contamination in environmental samples.
LITERATURE CITED
Brar, G.S. 2013. Residue dynamics of acephate, profenophos and triazophos in brinjal (Solanum melongena L.). M. Sc. Thesis. Dr. Y.S. Parmar University of Horticulture and Forestry, Nauni, Solan, H.P. India.
Chandrakala M.R., Srinivasan B.P., Niranjana K.V., Sujatha, K and Rajendra, H. 2022. Land suitability evaluation for pigeonpea in Semi-arid land, South Telangana plateau, India, using GIS, remote sensing and detailed survey. Communications in Soil Science and Plant Analysis. 53 (6): 675- 684.
Dodia, D.A., Prajapathi, B.G and Asharya, S. 2009. Efficacy of insecticides against pod borer, H. armigera, infesting pigeonpea. Journal of Food Legumes. 22(2): 35-38.
Gupta, S., Gajbhiye, V. T., Sharma, R. K and Gupta, R. K. 2011. Dissipation of cypermethrin, chlorpyriphos, and profenophos in tomato fruits and soil following application of pre-mix formulations. Environmental Monitoring and Assessment. 174: 337-345.
Lal, S.S and Katti, G. 1997. Podfly, Melanagromyza obtusa Malloch – A key pest of pigeonpea. Indian Institute of Pulse Research. Kanpur. 267.
Mukherjee, I., Kumar, A and Kumar, A. 2012. Persistence behaviour of combination mix crop protection agents in/on eggplant fruits. Bulletin of Environmental Contamination and Toxicology. 88: 338-343.
Naik, H.R., Rahul, C., Pallavi, M.S., Bheemanna, M., Rachappa, V., Pramesh, D., Anand, N and Udaykumar N. 2020. Determination of profenophos residues using LC-MS/MS and its dissipation kinetics in pigeonpea pods. Legume Research – An International Journal. 45 (11): 1372-1380.
Nigam, R.C., Pandaey, R.K., Yiwari, D.D and Katiyar, N.K. 2009. Persistence of endosulfan and profenophos in/on brinjal. Pesticide Research Journal. 21(2): 180-182.
Paras, N., Kumari, B., Yadav, P.R., Kathpal, T.S. 2005. Persistence and dissipation of ready mix formulations of insecticides in/on okra fruits. Environmental Monitoring and Assessment. 107(13):173-179.
Pramanik, S. K., Dutta, S., Bhattacharyya. J., Saha, T., Dey, P. K., Das, S., Bhattacharyya, A. 2005. Persistence of profenophos residue on tea under northeast Indian climatic conditions. Bulletin of Environmental Contamination and Toxicology. 74(4): 645-651.
Pratibha, G., Srinivas, I and Rao K.V. 2015. Impact of conservation agriculture practices on energy use efficiency and global warming potential in rainfed Pigeonpea-castor systems. European Journal of Agronomy. 66: 30–40.
Reddy, K.D., Reddy, K.N and Mahalingappa, P.B. 2007. Dissipation of fipronil and profenophos residues in chillies (Capsicum annum L.). Pesticide Research Journal. 19(1): 106- 107.
Renuka, S., Rajabaskar, D., Regupathy, A. 2006. Persistence and dissipation of profenophos 50 EC in cardamom. Indian Journal of Plant Protection. 34(2):165-167.
Sahoo, S.K., Kapoor, S.K and Singh, B. 2004. Estimation of residues of profenophos in/on tomato Lycopersicon esculentum Mill. Bulletin of Environmental Contamination and Toxicology. 72(5): 970-974.
Sharma, O. P., Rachappa, V., Suhas Y., Harischandra N., Gopali, J.B and Manish,R.S. 2018. Validation and implementation of principles of the Integrated Pest Management concept sustainability and current challenges in pest endemic pulse bowl of India. Indian Journal of Agricultural Sciences. 88(3): 474-81.
Shashi, B.V., Sreenivasa R.C., Swarupa R.S., Harinatha, R.A., Ravindranath, D., Aruna, M and Hymavathy, 2014. Dissipation dynamics and risk assessment of profenophos, triazophos and cypermethrin residues on brinjal for food safety. International Journal of Research in Agricultural Sciences. 2(2): 2348 – 3997.
Srivastava, C.P and Mohapatra, S.D. 2002. Field screening of pigeonpea genotypes for resistance to major insect pests. Journal of Applied Zoological Research. 13(2):202-203.
- Effect of Sowing Window on Nodulation, Yield and Post – Harvest Soil Nutrient Status Under Varied Crop Geometries in Short Duration Pigeonpea (Cajanus Cajan L.)
- Nanotechnology and Its Role in Seed Technology
- Challenges Faced by Agri Startups in Andhra Pradesh
- Constraints of Chcs as Perceived by Farmers in Kurnool District of Andhra Pradesh
- Growth, Yield Attributes and Yield of Fingermillet (Eleusine Coracana L. Gaertn.) as Influenced by Different Levels of Fertilizers and Liquid Biofertilizers
- Consumers’ Buying Behaviour Towards Organic Foods in Retail Outlets of Ananthapuramu City, Andhra Pradesh

