Amazing Plant Growth-Promoting Actinobacteria From Herbal Vermicompost
0 Views
V. SRINIVAS, A. SRAVANI, S. PRATYUSHA AND S. GOPALAKRISHNAN*
International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru 502 324, Telangana
ABSTRACT
Biological degradation and conversion of agricultural or herbal wastes by earthworms and microorganisms, called vermicomposting, is becoming a favored method of recycling biodegradable wastes. Application of vermicompost prepared from the herbals not only benefits crop plants, as it contains beneficial microorganisms, that help the plants to mobilize and acquire nutrients, but also promotes plant growth and inhibits phytopathogens. The use of plant growth-promoting (PGP) micro- organisms for sustainable agriculture has increased tremendously in many parts of the world as it is widely reported to enhance the plant growth and yield of agriculturally important crops. PGP microorganisms facilitate the plant growth either by direct means (such as nitrogen fixation, phosphate solubilization, iron chelation and phytohormones production) or by indirect means such as inhibition of phytopathogens. Actinobacteria are gram positive filamentous bacteria that are known to produce antibiot- ics effective against fungal plant pathogens and possess PGP traits. This article gives an outline of how actinobacteria isolated from such herbal vermi compost at International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) were exploited for crop production and crop protection.
KEYWORDS: Actinobacteria, PGP microorganisms, vermicompost
INTRODUCTION
Vermicomposting, a decomposition process of organic material through the use of earthworms and microbes, got more attraction in sustainable agriculture due to low cost technology, utilization of wide range of initial substrate and eco-friendly process (Prabha, 2009). Vermicompost has substantial quantities of macro- and micro-elements and growth-promoting substances with desirable soil physical properties (Aira et al., 2007). Use of vermicompost as total/partial substitute for chemical fertilizers in potting media or as soil amendments under field conditions demonstrated to produce lush growth on variety of crops including cereals, fruits, vegetables and horticultural crops (Hameeda et al., 2006; Perner et al., 2006; Nath and Singh, 2009). All these beneficial actions of vermicompost are indirectly contributed by the microbial partners such as bacteria, fungi and actinomycetes that resides in the gut of earthworms.
Application of vermicompost was found to increase the growth of vegetables, fruits, flowers and agriculturally important food crops not only by their macro and micro- element composition of the vermicast but also by their growth-promoting hormones such as auxins, gibberellins, cytokinins of microbial origin and enzymes and humic acids (Edwards, 1998). Further, occurance of microbial population in the vermicompost acts as bio-control agents due to the production of antibiotics and secretion of extracellular enzymes such as chitinase, protease and lipase which causes the lysis of fungal and bacterial phytopathogens. Vermicompost is an important source of antagonistic actinobacteria or bacteria against phytopathogens such as Botrytis cinerea, Fusarium oxysporum, Rhizoctonia spp., Phytophthora spp. and Plasmodiophora brassicae and also affects growth of insect pests such as leafhoppers, aphids, , mealy bugs, caterpillars and beetles. Application of vermicompost has been widely demonstrated and reported to manage plant diseases and insect pests in barley, balsam, pea, clover, cabbage, cucumber, grapes, tomatoes, radish and strawberry under field conditions.
Actinobacteria are a group of Grampositive bacteria, with a high G + C content belonging to the order Actinomycetales, found most commonly in soil, compost, fresh and marine water and play an important role in the PGP, plant protection, decomposition of organic materials and produce secondary metabolites of commercial interest. Actinomycetes, in the rhizosphere, help in enhancing root growth, shoot growth, plant hormone concentrations, nitrogen fixation, the solubilisation of minerals and the suppression of plant pathogens. Further, there is a growing interest in the use of secondary metabolites, such as toxins, proteins, hormones, vitamins, amino acids and antibiotics, from microorganisms, particularly from actinobacteria, for the control of plant pathogens as these are readily degradable, highly specific and less toxic to nature. Beneficial PGP and potential of actinobacteria was well documented in tomato, wheat, rice, bean, chickpea and pea. ICRISAT isolated number of such PGP actinobacteria from various herbal composts characterized for in-vitro PGP properties and further demonstrated for their usefulness under greenhouse and field conditions in rice, sorghum, chickpea and pigeonpea. This article gives an outline of how actinobacteria isolated from such herbal vermicompost at ICRISAT were demonstrated for crop production and crop protection and thus can be further exploited under farmer field conditions.
MATERIAL AND METHODS
Isolation of actinobacteria from herbal vermicompost
Foliages of 25 different herbals (Jatropha curcas, Annona squamosa, Parthenium hysterophorus, Oryza sativa, Gliricidia sepium, Adhatoda vasica, Azadirachta indica, Capsicum annuum, Calotropis gigantea, Calotropis procera, Datura metal, Allium sativum, Zingiber officinale, Ipomoea batatas, Momordica charantia, Moringa oleifera, Argyranthemum frutescens, Nerium indicum, Allium cepa, Curcuma aromatica, Pongamia pinnata, Abacopteris multilineata, Nicotiana tabacum, Tridax procumbens and Vitex negundo) were collected from the ICRISAT farm and composted with earthworms (Eisenia foetida). When the herbal compost was ready in about two months, 10 grams of it were used for isolation of actinobacteria as per the standardized protocols. No actinobacteria was found in the compost prepared from Datura foliage, while all the other vermicomposts contained actinobacteria in the range of 6.3″7.7 Log10 (CFU) populations with an exception of tobacco compost, which contained only 2.0 Log10 populations. Maximum diversity of actinobacteria was found in vermicomposts prepared with Chrysanthemum, Oleander and Pongamia. A total of 137 actinobacteria, the most prominent ones (the ones which were found abundantly, produced pigments and inhibited the adjacent colonies) in the starch casein agar plate, were isolated and further screened for their antagonistic potential against important diseases of chickpea such as Fusarium wilt, collar rot, dry root rot and Botrytis gray mold and sorghum such as charcoal rot by dual culture assay. Based on these results, a total of 19 actinobacterial isolates (CAI- 13, CAI-17, CAI-21, CAI-24, CAI-26, CAI-68, CAI-78, CAI-85, CAI-93, AI-121, CAI-127, CAI-140, CAI-155, KAI-26, KAI-27, KAI-32, KAI-90, KAI-180 and MMA- 32) were shortlisted for characterization of their enzymatic activities and secondary metabolite production.
Enzymatic activities and secondary metabolite production by the actinomycete isolates
Siderophore production
Siderophore production was determined according to the methodology described by Schwyn and Neilands (1987). Actinomycetes were streaked on chrome azurol S (CAS) agar media and incubated at 28 ± 2°C for four days. When the actinomycetes consume iron, present in the blue-colored CAS media, orange halos are produced around the colonies, which indicate the presence of siderophores. Observations were recorded on a 0″4 rating scale as follows: 0 = no change; 1 = positive; 2 = halo zone of 1″3 mm; 3 = halo zone of 4″6 mm and 4 = halo zone of 7 mm and above.
Cellulase production
The standardized protocols of Hendricks et al. (1995) were used to evaluate the cellulase production. Actinomycetes were streaked on cellulose Congo red agar media and incubated at 28 ± 2°C for four days. The plates were observed for halo zone around the actinomycete colonies, which indicate the presence of cellulase. Observations were recorded on a 0″4 rating scale as follows: 0 = no change; 1 = positive; 2= halo zone of 1″3 mm; 3 = halo zone of 4″6 mm and 4 = halo zone of 7 mm and above.
Hydrocyanic acid (HCN) production
HCN was estimated qualitatively by the sulfocyanate colorimetric method (Lorck, 1948). The actinomycetes were grown in Bennett agar amended with glycine (4.4 g l-1). One sheet of Whatman filter paper no. 1 (8 cm diameter) was soaked in 1% picric acid (in 10% sodium carbonate; filter paper and picric acid were sterilized
separately) for a minute and stuck underneath the Petri dish lids. The plates were sealed with Parafilm and incubated at 28 ± 2°C for four days. Development of reddish brown color on the filter paper indicated positive for HCN production. Observations were recorded (by a panel of three observers) on a 0″3 rating scale (they were rated based on the intensity of the reddish brown color) as follows: 0 = no color change; 1 = light reddish brown; 2 = medium reddish brown and 3 = dark reddish brown.
Indole acetic acid (IAA) production
It was done as per the protocols of Patten and Glick (1996). The actinomycetes were grown in starch casein broth supplemented with L-tryptophan (1 ì g ml-1) for four days. At the end of the incubation, the cultures were centrifuged at 10,000 g for 10 min and the supernatants collected. One ml of this culture filtrate was allowed to react with 2 ml of Salkowsky reagent (1 ml of 0.5 M FeCl3 in 50 ml of 35% HClO4) at 28 ± 2 oC for 30 min.
At the end of the incubation, development of pink color indicated the presence of IAA. Quantification of IAA was done my measuring the absorbance in a spectrophotometer at 530 nm. A standard curve was plotted to quantify the IAA (ìg ml-1) present in the culture filtrate.
Physiological traits of the prominent actinobacteria
The selected 19 potential antagonistic actinobacteria were able to grow in NaCl up to 12%, pH values between
5 and 12 (acidic to highly alkaline) and temperatures between 20 and 40oC. However, the optimum conditions for good growth were 0″4% NaCl, pH values of 7″12 and temperatures of 20″30oC.
On growth-promotion and yield enhancement traits in grain legume pigeonpea
On pigeonpea, in the field conditions, 3 out of 19 strains (CAI-21, CAI-26 and MMA-32) of actinobacteria. were earlier reported to enhance nodule number, nodule weight, plant biomass, total dry matter and grain yield In the current study, the remaining sixteen actinobacteria strains were evaluated for their PGP in the pigeonpea under field conditions at International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, India. The trial was conducted in a randomized complete block design (RCBD) with three replications. The plot size was 4 rows of 1.2 m long with a row-to-row spacing of 60 cm and a plant-to-plant spacing of 10 cm. Pigeonpea seeds were sown in the field at a depth of 5 cm. The seeds were treated with the sixteen actinobacteria. strains (containing 108 CFU mL-1) separately at sowing and at every 15 days interval up to 45 DAS. A negative control of only water was also maintained. Once the crops reach their physiological maturity stage (~30 days), the effect of actinobacteria. on the agronomic performance of the pigeonpea were evaluated. Later, at harvest, the effect of the sixteen actinobacteria. on agronomic performance and yield potential of pigeonpea under field conditions were also recorded.
RESULTS AND DISCUSSION
Enzymatic activities and secondary metabolite production by the prominent actinobacteria
When the 19 potential antagonistic actinobacteria (CAI-13, CAI-17, CAI-21, CAI-24, CAI-26, CAI-68, CAI-78, CAI-85, CAI-93, AI-121, CAI-127, CAI-140, CAI-155, KAI-26, KAI-27, KAI-32, KAI-90, KAI-180 and MMA-32) were evaluated further for their enzymatic activities and secondary metabolite production, all the isolates produced hydrocyanic acid (HCN), indole acetic acid (IAA; except KAI-90), siderophore (except CAI-78 and KAI-180) and cellulase (except CAI-26). Further, eleven of the isolates also produced lipase (except CAI- 21, CAI-26, CAI-85, CAI-93, CAI-121, KAI-27, KAI-32and MMA-32), fourteen produced chitinase (except CAI- 21, CAI-68, CAI-78, CAI-85 and CAI-140) and nine produced â-1-3-glucanase (except CAI-21, CAI -24, CAI- 26, CAI-93, CAI-121, CAI-127, KAI-32, KAI-90, KAI-180 and MMA-32). Hence, it was concluded that the 19 actinobacteria had rich enzymatic activities and secondary metabolite production capabilities (Gopalakrishnan et al., 2011a, 2013, 2015 (Table .1).
Physiological traits of the prominent actinobacteria
The physiological traits and usefulness of these selected actinobacteria on different crops from various studies at ICRISAT, Patancheru have been presented in Table 2
On growth-promotion and yield enhancement traits in grain legumes pigeonpea
The results showed that at 30 DAS, all the sixteen actinobacteria strains have significantly enhanced number of nodules (up to 80%), nodule weight (up to 77%), root weight (up to 30%) and shoot weight (up to 19%) of pigeonpea over the un-inoculated control (Table 3). At harvest, these selected actinobacteria have significantly enhanced the pigeonpea stover and pod weight (27% and 28% respectively), number of pods (up to 38%), seed weight and number (40% and 45% respectively), grain and stover yield (54% and 80% respectively) over the un-inoculated control. Of the 16 selected Streptomyces isolates, PGP traits were found maximum in KAI-180 followed by CAI-140, CAI-155 and CAI-17 over the un- inoculated control (Table. 4).
The selected 19 actinobacteria were also demonstrated for their biofortification traits in chickpea under field conditions. All the 19 actinobacteria were found to significantly increase minerals such as Fe, Zn, Ca, Cu, Mn and Mg over the un-inoculated control. The per cent increase might be due to the production of siderophore-producing capacity of the tested actinobacteria, which was confirmed in our previous studies by q-RT PCR on siderophore genes expressing up to 1.4 to 25 fold increased relative transcription levels (Sathya et al., 2016;). Five of the 19 actinobacteria (CAI- 24, CAI-121, CAI-127, KAI-32 and KAI-90) were demonstrated for their antagonistic potential against Fusarium wilt, caused by Fusarium oxysporum f. sp. ciceri (FOC), under greenhouse and field conditions in chickpea (Gopalakrishnan et al., 2011a). Another set of six actinobacteria ( CAI-17, CAI-21, KAI-26, KAI-27, MMA-32 and SAI-13) were demonstrated for their antagonistic potential against charcoal rot, caused by
Macrophomina phaseolina, under greenhouse and field conditions in sorghum (Gopalakrishnan et al., 2020a). The secondary metabolite responsible for the inhibition of the M. phaseolina was also purified and identified (Gopalakrishnan et al., 2020a).
One of the selected 19 actinobacteria (Streptomyces sp. CAI-155) was demonstrated to have entomopathogenic potential against Helicoverpa armigera, Spodoptera litura and Chilo partellus, important insect pests of chickpea and sorghum (Vijayabharathi et al., 2014). The active secondary metabolite (from the culture filtrates of CAI-155) was purified and identified as N-(1-(2, 2-dimethyl-5-undecyl- 1, 3-dioxolan-4-yl)-2-hydroxyethyl) stearamide by NMR and mass spectral studies. The purified metabolite resulted in 70-78 per cent mortality in 2nd instar larvae of H. armigera in a diet impregnation assay, detached leaf assay and greenhouse assay (Gopalakrishnan et al., 2016).
Washings of vermi-compost called ‘bio-wash’ was also found to have inhibitory activity against phytopathogens and insect pests particularly against H. armigera, S. litura and C. partellus (Gopalakrishnan et al., 2011b). Among different bio-wash, bio-wash of Jatropha curcas, Annona squamosa and Parthenium hysterophorus marked their fungicidal activity with their crude extract and partially purified extracts on FOC, S. rolfsii and M. phaseolina. These studies suggest that vermicompost and bio-wash has the potency to promote
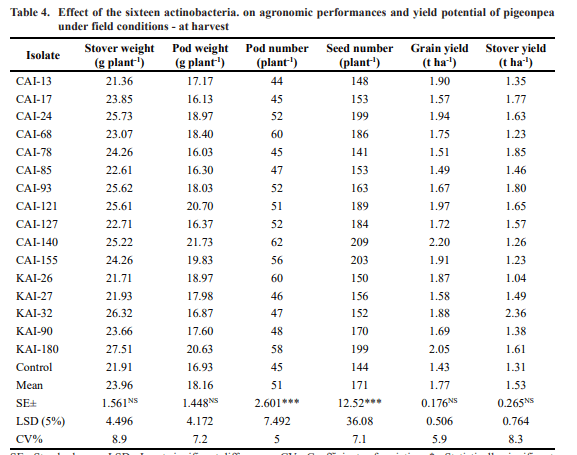
SE= Standard error; LSD= Least significant differences; CV= Coefficients of variation; *= Statistically significant at 0.05, **= Statistically significant at 0.01, ***= Statistically significant at 0.001, NS= Not significant
plant growth, control the infectious diseases and restrict the pest attack.
Complete genome sequence of the prominent actinobacteria
Out of the 19 prominent actinobacteria, the genome sequences of 16 actinobacteria (CAI-17, CAI-21, CAI- 24, CAI-68, CAI-78, CAI-85, CAI-93, CAI-121, CAI-127, CAI-140, CAI-155, KAI-26, KAI-27, KAI-90, KAI-180
and MMA-32) have been decoded by whole genome sequences (WGS). The genome assemblies of the 16 actinobacteria strains ranged from 6.8 Mb to 8.31 Mb, with a GC content of 72 to 73%. The extent of sequence similarity in 16 actinobacteria strains showed 70 to 85% common genes to the closest publicly available Streptomyces genomes. Genome assemblies of the 16 actinobacteria strains have also provided genes involved in key pathways related to PGP and biocontrol traits such as siderophores, IAA, hydrocyanic acid, chitinase and cellulase (Gopalakrishnan et al., 2020b).
CONCLUSION
Vermicompost has been extensively used in organic agriculture not only for its beneficial effects on soil biota and structure, but also for its ability to promote plant growth and inhibit plant pathogens. Vermicompost contains complex group of beneficial microorganisms, which directly or indirectly contribute to its beneficial properties in enhancing the soil health, plant growth and indeed the agricultural productivity. The present chapter narrates the successful selection of 19 prominent actinobacteria isolated from 25 different herbal vermicompost and their potentials in integrated pest, disease and nutrient management. The 19 actinobacteria have been demonstrated extensively for their PGP potentials in chickpea, pigeonpea, sorghum and rice by significantly enhancing nodulation, nitrogen fixation, crop productivity and root development. As biocontrol agents, they are effectively active against Fusarium wilt in chickpea, charcoal rot in sorghum and entomopathogenic towards Helicoverpa armigera, Spodoptera litura and Chilo partellus. It is concluded that the selected 19 actinobacteria could be exploited as PGP and biocontrol agents in furthering the use of eco-friendly biological products. However, further experiments are needed to determine the effectiveness of these isolates under different field conditions and to understand the nature of interaction with other soil native microflora and fauna and the host plant.
ACKNOWLEDGMENTS
We thank PVS Prasad for his significant contribution in the laboratory, greenhouse and field studies. This work has been undertaken as part of the CGIAR Research Program on Grain Legumes Dry Land Cereals. ICRISAT is a member of the CGIAR Consortium.
LITERATURE CITED
Aira, M., Monroy, F. and Dominguez, J. 2007. Earthworms strongly modify microbial biomass and activity triggering enzymatic activities during vermicomposting independently of the application rates of pig slurry. Science of Total Environment. 385: 252-261.
Edwards, C.A. 1998. The use of earthworms in processing organic wastes into plant growth media and animal feed protein. In: Earthworm Ecology (ed.) Edwards CA, CRC press: Boca Raton, Florida. 327-354.
Gopalakrishnan, S., Pande, S., Sharma, M., Humayun, P., Kiran, B.K., Sandeep, D., Vidya, M.S., Deepthi, K and Rupela, O. 2011a. Evaluation of actinomycete isolates obtained from herbal vermicompost for biological control of Fusarium wilt of chickpea. Crop Protection. 30: 1070–1078.
Gopalakrishnan, S., Ranga Rao, G.V., Humayun, P., Rao, V.R., Alekhya, G., Jacob, S., Deepthi, K., Vidya, M.S., Srinivas, V., Mamatha, L and Rupela, O. 2011b. Efficacy of botanical extracts and entomopathogens on control of Helicoverpa armigera and Spodoptera litura. African Journal of Biotechnology. 10(73): 16667-16673.
Gopalakrishnan, S., Srinivas, V., Shravya, A., Prakash, B., Ratnakumari, B., Vijayabharathi, R and Rupela,
O. 2013. Evaluation of Streptomyces spp. for their Plant growth-promoting traits in rice. Canadian Journal of Microbiology. 59: 534-539.
Gopalakrishnan, S., Srinivas, V., Alekhya, G and Prakash, B. 2015. Effect of plant growth-promoting Streptomyces sp. on growth promotion and grain yield in chickpea (Cicer arietinum L). 3 Biotech. 5: 799–806.
Gopalakrishnan, S., Rajendran, V., Arumugam, S., Sharma, H.C., Vadlamudi, S., Bhimineni, R.K., Gonzalez, S.V., Melø, T.M and Simic, N. 2016. Insecticidal activity of a novel fatty acid amide derivative from Streptomyces species against Helicoverpa armigera. Natural Product Research. 30: 2760-2769.
Gopalakrishnan, S., Sharma, R., Srinivas, V., Naresh, N., Mishra, S.P., Ankati, S., Pratyusha, S., Govindaraj, M., Gonzalez, S.V., Nervik, S and Simic, N. 2020a. Identification and Characterization of a Streptomyces albus Strain and Its Secondary Metabolite Organophosphate against Charcoal Rot of Sorghum. Plants. 9: 1727.
Gopalakrishnan, S., Thakur, V., Saxena, R.K., Vadlamudi, S., Purohit, S., Kumar, V., Rathore, A., Chitikineni, A and Varshney, R.K. 2020b. Complete genome sequence of sixteen plant growth promoting Streptomyces strains. Scientific Reports. 10(1): 10294.
Hameeda, B., Harini, G., Rupela, O.P., Wani, S.P. and Reddy, G. 2006. Growth promotion of maize by phosphate solubilizing bacteria isolated from composts and macrofauna. Microbiological Research. 163: 234-242.
Hendricks, C.W., Doyle, J.D., Hugley, B., 1995. A new solid medium for enumerating cellulose-utilizing bacteria in soil. Applied and Environmental Microbiology. 61: 2016-2019.
Lorck, H., 1948. Production of hydrocyanic acid by bacteria. Physiologia Plantarum. 1: 142-146.
Nath, G. and Singh, K. 2009. Utilization of vermiwash potential on certain summer vegetable crops. Journal of Central European Agriculture. 10: 417-426.
Patten, C and Glick, B.R. 1996. Bacterial biosyntehsis of indole-3-acetic acid. Canadian Journal of Microbiology. 42: 207-220.
Perner, H., Schwarz, D and George, E. 2006. Effect of mycorrhizal inoculation and compost supply on growth and nutrient uptake of young leek plants grown on peat-based substrates. Horticultural Science. 4: 628-632.
Prabha, M.L. 2009. Waste management by vermi technology. Indian Journal of Environmental Protection. 29: 795-800.
Sathya, A., Vijayabharathi, R., Srinivas, V and Gopalakrishnan, S. 2016. Plant growth-promoting actinobacteria on chickpea seed mineral density: an upcoming complementary tool for sustainable biofortification strategy. 3 Biotech. 6: 138.
Schwyn, B., Neilands, J.B. 1987. Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry. 160: 47-56
Vijayabharathi, R., Kumari, B.R and Gopalakrishnan, S. 2014. Microbial agents against Helicoverpa armigera: Where are we and where do we need to go? African Journal of Biotechnology. 13(18): 1835- 1844.
- Genetic Diversity Analysis of 64 Maize Inbred Lines for Yield Traits Using D2 Statistics and Principal Component Analysis
- Effect of Border Crops on Activity of Predatory Fauna In Blackgram (Vigna Mungo L.)
- Effect of Organic Nutrient Management Practices on Growth and Yield of Foxtail Millet
- Species Diversity of Sugarcane Shootborers in Major Sugarcane Growing Districts of Andhra Pradesh
- Faunistic Studies on Economically Important Lepidopterans in Different Field Crops of Tirupati District
- Production Potential of Sweet Corn as Influenced by Organic Manures and Foliar Nutrition

