Morphological Basis for Resistance Against Whitefly in Castor
0 Views
B. KAMESWARA RAO*, A. RAJESH, P. DURAIMURUGAN, G. MOHAN NAIDU,G. NARAYANA SWAMY AND M. RAJASRI
Department of Entomology, S.V. Agricultural College, ANGRAU, Tirupati-517 502.
ABSTRACT
Castor (Ricinus communis) is a perennial shrub and a vital industrial non-edible oilseed crop in India. The castor whitefly, is a polyphagous tropical insect pest, feeds undersurface of leaves. Among twenty-five castor genotypes evaluated for morphological host plant traits, viz., bloom, stem color, leaf thickness, and chlorophyll content against whitefly, nine genotypes showed resistant reaction, seven were moderately resistant, six moderately susceptible and three genotypes showed susceptible reaction. The lowest mean nymphal and pupal population of whitefly was observed in the genotypes, RG-2466 (20.69 plant-1) followed by ACH-2008 (20.74 plant-1), whereas the highest whitefly population was observed in YRCH-1 (409.98 plant-1) followed by M-574 (407.40 plant-1). The castor genotypes with zero and single bloom were found to be resistant to whitefly, while the genotypes with triple bloom showed susceptible reaction. A significant negative correlation was observed between chlorophyll content and whitefly population in different castor genotypes, whereas, non-significant negative correlation observed between stem color, leaf thickness and whitefly population.
KEYWORDS: Castor, Whitefly, Bloom, Leaf thickness, Chlorophyll content.
INTRODUCTION
Castor (Ricinus communis) a perennial shrub from the Euphorbiaceae family is a vital industrial non-edible oilseed crop (Ramanjaneyulu et al., 2017: Snehalatha et al., 2024), cultivated in over 30 countries (Agyenim- Boateng et al., 2018). India is the world’s largest producer of castor, contributing over 85% of global production (FAO, 2020). The cultivation and yield of castor are frequently hampered in India by several insect pests, of which the whitefly has become a serious threat in recent years (Ranganath et al., 2021: Ranga et al., 2022). The castor whitefly is a polyphagous, multivoltine tropical insect pest feeding on the leaves of a wide range of host species including castor, cowpea, cotton, pumpkin, and sweet potato (Bink-Moenen., 1983). Additionally, it has been identified as a vector of Tomato Yellow Leaf Curl Begomovirus (Idriss et al., 1997).
Chemical control through insecticides has proven to be ineffective against this pest, as both its nymphal and adult stages feed and complete their life cycle on the undersides of leaves, making them difficult to target (Haran et al., 2024). Hence, the identification and development of sources of resistance to this insect will serve as a key for the sustainable management in long run and also limit the indiscriminate use of pesticides. The present study aims to identify sources of resistance against the whitefly in various castor genotypes. Morphological plant traits, such as bloom intensity, stem color, leaf thickness and chlorophyll content, were analyzed for their correlation with whitefly population density to determine potential plant characteristics that contribute to resistance mechanisms.
MATERIAL AND METHODS
The experimental material comprised of twenty- five castor genotypes, procured from the Agricultural Research Station, Ananthapuramu, Andhra Pradesh and the ICAR-Indian Institute of Oilseeds Research, Hyderabad, Telangana, were screened against castor whitefly under open field conditions at the Agricultural research station, Ananthapuramu, Andhra Pradesh during the kharif , 2024. The experiment was laid out in a Randomized Block Design (RBD) with two replications by following infester row technique with susceptible (DCH-519) and resistant (DPC-9) checks. The size of the plot was 6×1m with an inter row spacing of 90 cm and an intra row spacing of 60 cm. The resistant and susceptible checks were planted after every six test genotypes. All recommended agronomic practices were followed, except for pest management.
Five plants were randomly selected from each genotype and tagged to assess the population of whitefly
Table 3.1. Castor genotypes categorized based on the scoring scale (Haran et al., 2024)
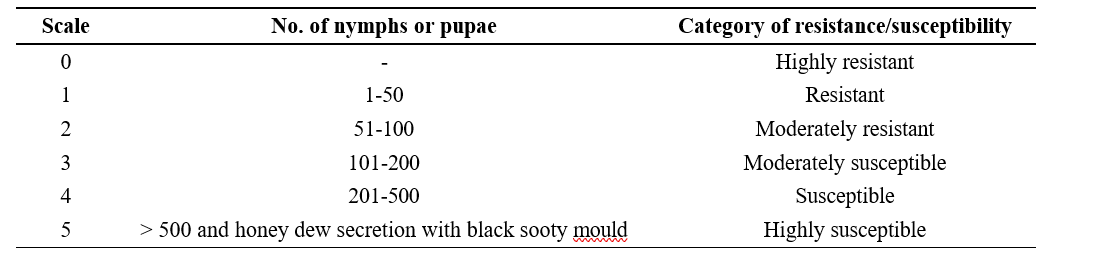
nymphs and pupae in castor. Observations commenced at 4 Weeks After Sowing (WAS) onwards to till 20 WAS and recorded at weekly intervals. The data on whitefly nymphs and pupae were recorded on under surface of three leaves of castor genotypes from the top, middle, and bottom portion of the main shoot.
Bloom
Castor plants have epicuticular wax called bloom. The genotypes were graded as zero, single, double and triple bloom based on the following observations as standardized by ICAR-IIOR. Castor genotypes were categorized based on the bloom and wax coating which are described as follows; The ones with wax coating on all parts of the plant were classified as triple bloom whereas the ones with bloom on the stem as well as petioles, on the lower side of the leaf, but not the upper surface were called double bloom on the other hand genotypes with the wax coating on the stem were described as single bloom and the genotypes without wax coating on plant were known as no bloom genotypes.
Stem color:
The stem color of the main shoot of castor genotypes was observed at 60 DAS on five randomly selected plants in each genotype and recorded as green, red and mahogany.
Leaf thickness:
The leaf thickness of three leaves (top, middle and bottom) was measured on three randomly selected plants of each genotype. The leaf thickness was measured midway between the margin and the midrib at the widest part of the leaf and also across three different locations on the leaf using a digital screw gauge (micrometer) and was expressed in units of mm.
Chlorophyll content:
The chlorophyll content of castor genotypes was assessed using a SPAD meter on three leaves from the top, middle and bottom sections of the main stem and expressed as SCMR (SPAD Chlorophyll Meter Readings).
Statistical Analysis
The data on incidence of whiteflies per three leaves per plant collected at weekly intervals on different castor genotypes was subjected to square root transformations, Analysis of Variance (ANOVA) and Duncan’s Multiple Range Test (DMRT). Similarly, the data on morphological characters of different castor genotypes was subjected to square root transformations, Analysis of Variance (ANOVA), Duncan’s Multiple Range Test (DMRT) and correlation with the help of SPSS statistical package.
RESULTS AND DISCUSSION
Incidence of whitefly on castor genotypes was recorded from 4 Weeks After Sowing (WAS) onwards to till 20 WAS as the pest population was negligible till 4 WAS. The incidence of whitefly on castor genotypes observed at 8 WAS ranged from 16.25 to 262.50 nymphs and pupae per three leaves plant⁻¹ respectively. The highest number of whitefly nymphs and pupae were recorded in the genotype M-574 (262.50 plant⁻¹) followed by DCH-519 (259.50 plant⁻¹) and YRCH-1 (253.00 plant⁻¹). The lowest whitefly population was recorded in ACH-2008 (16.25 plant⁻¹), followed by the resistant check, DPC-9 (20.40 plant⁻¹) and RG-2466 (21.95 plant⁻¹). At 12 WAS the mean whitefly population ranged from 19.30 to 558.70 nymphs and pupae per three leaves plant⁻¹. The lowest number of whitefly nymphs and pupae was
Table 4.1. Incidence of castor whitefly on different test genotypes during kharif, 2024
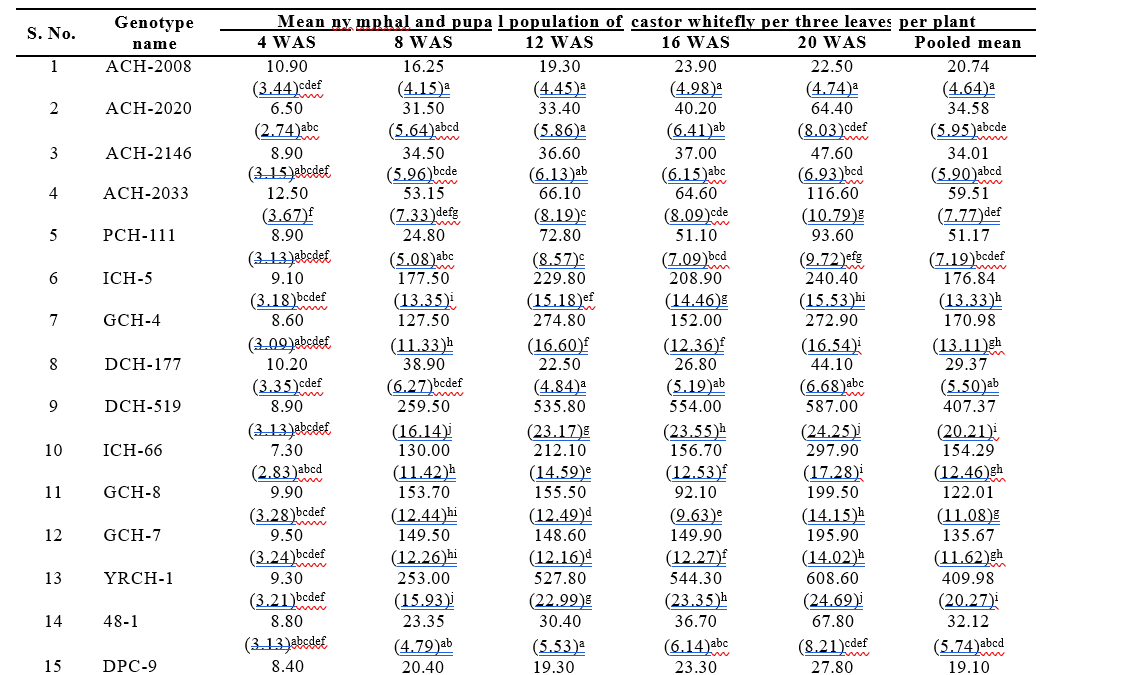
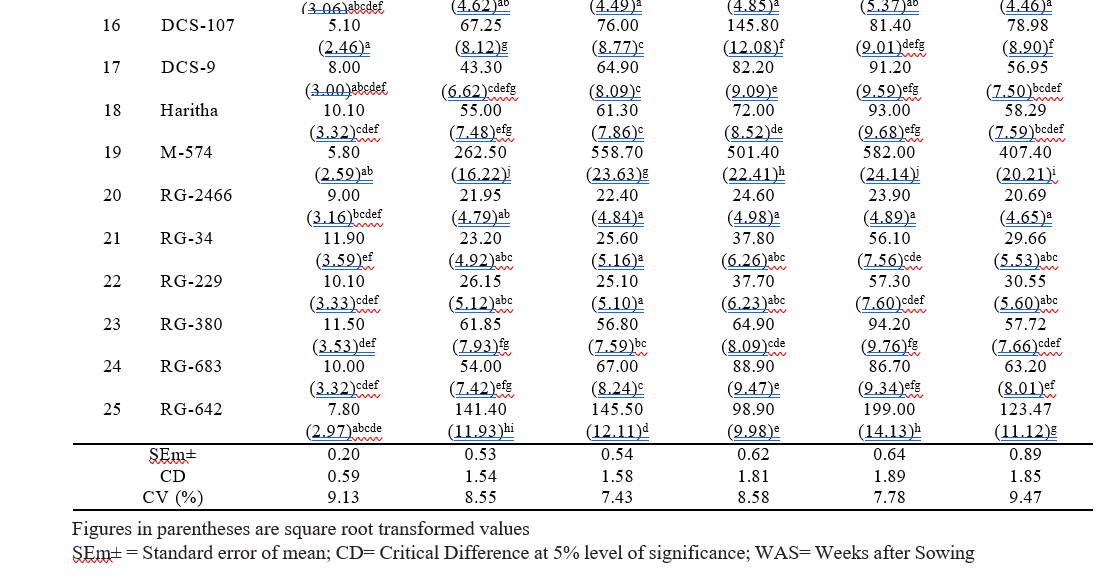
Table 4.2. Reaction of different castor genotypes to whitefly population during kharif 2024
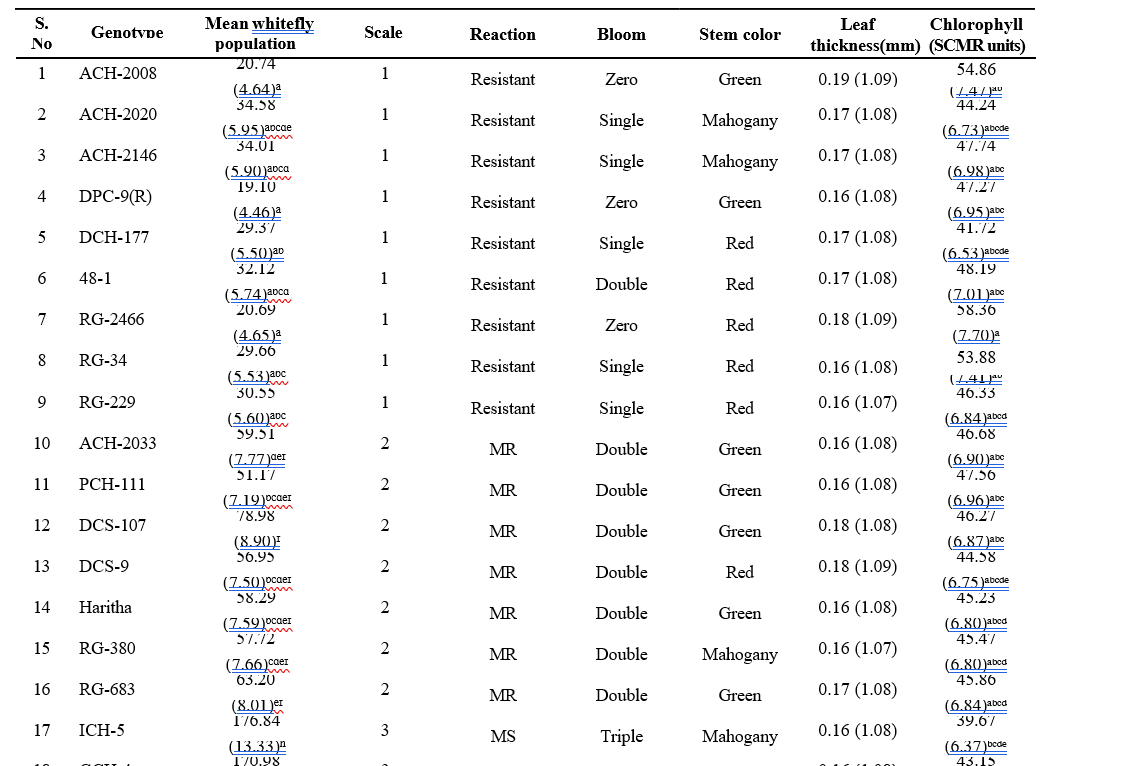
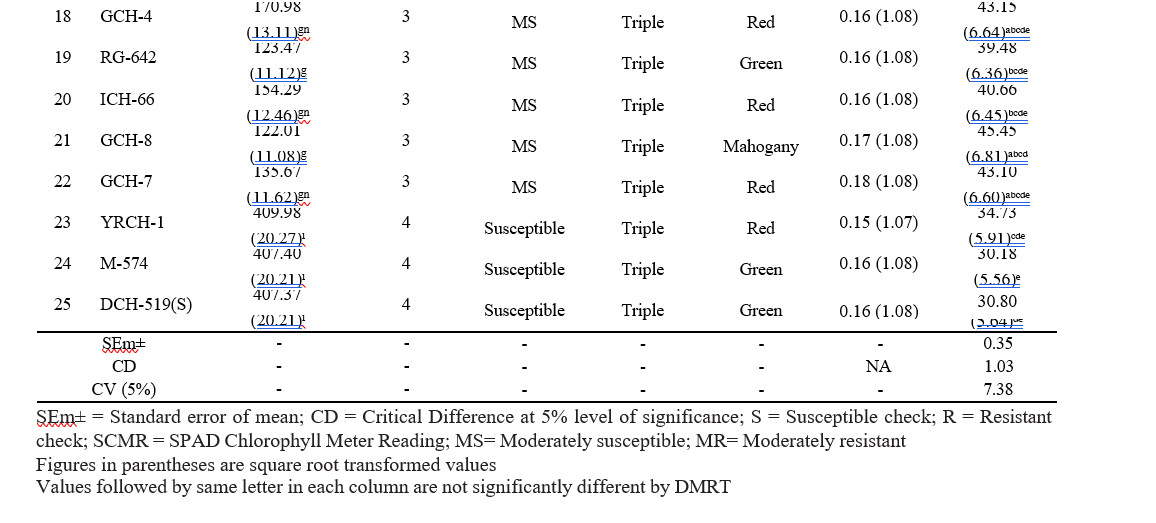
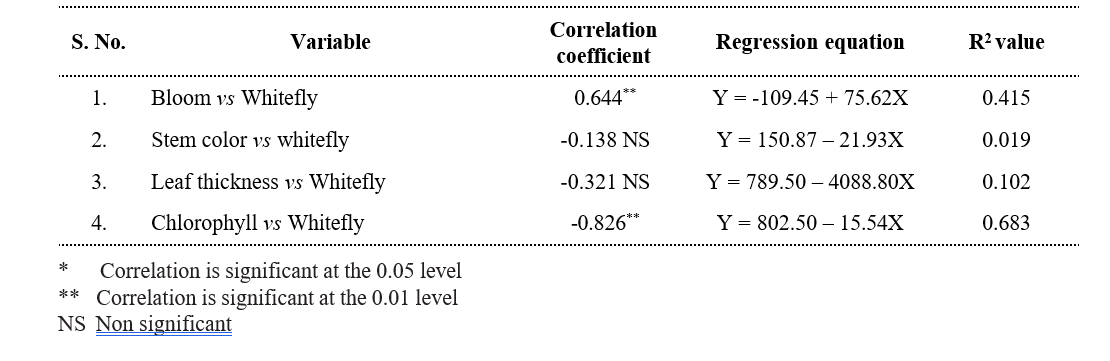
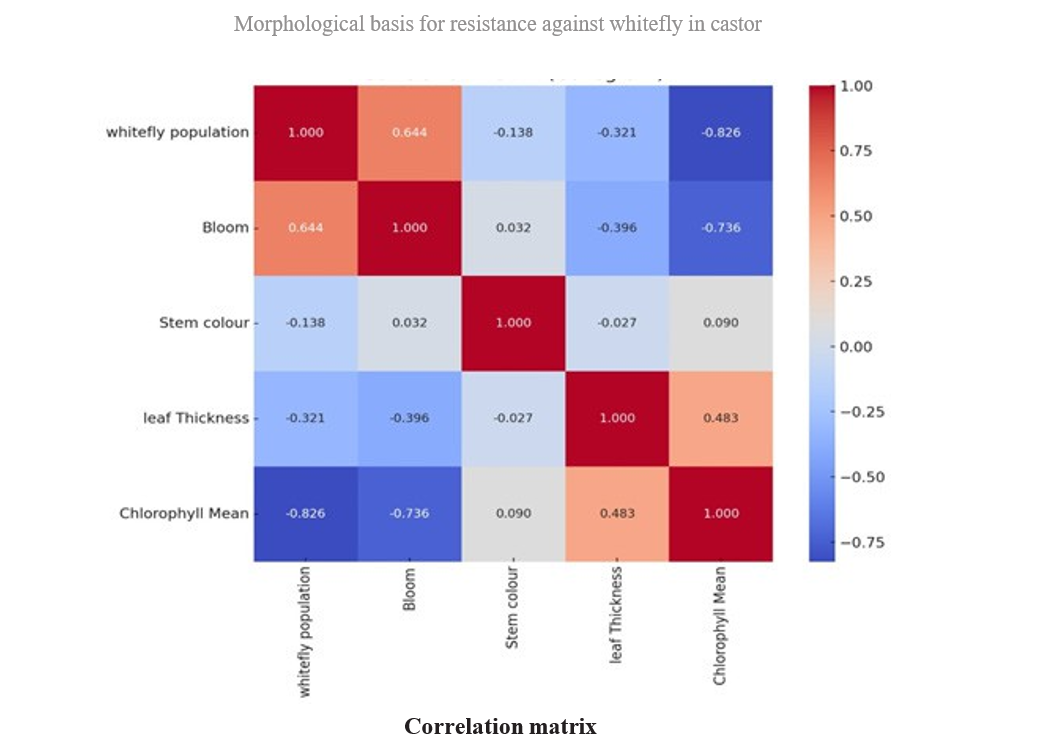
Table 4.3. Correlation matrix of relationship between biophysical constituents and whitefly incidence on different castor genotypes
recorded in the genotype ACH-2008 (19.30 plant⁻¹) followed by RG-2466 (22.40 plant⁻¹) and DCH-177 (22.50 plant⁻¹) which demonstrated a relatively higher level of resistance. Conversely, the highest whitefly nymphs and pupal population was observed in M-574 (558.70 plant⁻¹) followed by DCH-519 (535.80 plant⁻¹) and YRCH-1 (527.80 plant⁻¹) which were statistically on par.
At 16 WAS, the susceptible check DCH-519 continued to harbour a high population of whiteflies nymphs and pupae (554.0 plant⁻¹) followed by YRCH-1 (544.3 plant⁻¹) and M-574 (501.4 plant⁻¹) which were on par with each other. The genotypes viz., ACH-2008, RG-2466, DCH-177 and 48-1 were found least preferred by the whitefly with 23.30, 23.90, 24.60, 26.80, and 36.70 nymphs and pupae plant⁻¹, respectively and were not significantly different from each other. A similar trend was observed in the whitefly population (nymphs and pupae) on castor genotypes at 20 WAS and results revealed that lowest population was recorded on genotype ACH-2008 (22.50 plant⁻¹), RG-2466 (23.90 plant⁻¹), DCH-177 (44.10 plant⁻¹) and ACH-2146 (47.60 plant⁻¹). The resistant check, DPC-9, also recorded a relatively low population of 27.80 nymphs and pupae plant⁻¹. Whereas significantly high whitefly population was observed on YRCH-1 (608.60 plant⁻¹) followed by susceptible check DCH-519 (587.00 plant⁻¹) and M-574 (582.00 plant⁻¹).
The pooled mean of whitefly population (nymphs and pupae) recorded on different castor genotypes during the crop period ranged from 19.10 to 409.98 nymphs and pupae per three leaves plant⁻¹. The lowest whitefly population was observed in the genotypes RG-2466 (20.69 plant⁻¹) followed by ACH-2008 (20.74 plant⁻¹) and DCH-177 (29.37 plant⁻¹). The resistant check DPC-9 recorded the 19.10 nymphs and pupae plant⁻¹. The next best genotypes viz., RG-34 (29.66 plant⁻¹) and RG-229 (30.55 plant⁻¹) were statistically on par with 48-1 (32.12 plant⁻¹), ACH-2146 (34.01 plant⁻¹) and ACH-2020 (34.58 nymphs & pupae plant⁻¹). The genotypes, PCH-111 (51.17 plant⁻¹), DCS-9 (56.95 plant⁻¹), Haritha (58.29 plant⁻¹) and RG-380 (57.72 plant⁻¹) were statistically on par with each other, while the genotype ACH-2033 (59.51 plant⁻¹) was statistically on par with RG-683 (63.20 plant⁻¹) and DCS-107 (78.98 plant⁻¹). The highest whitefly population was recorded in YRCH-1 (409.98 plant⁻¹) followed by M-574 (407.40 plant⁻¹) and DCH-519 (407.37 plant⁻¹), which were on par and statistically significant with the remaining genotypes. The next most preferred genotypes by whitefly were ICH-5, GCH-4, ICH-66, GCH-7, RG-642 and GCH-8 with a population of 176.84, 170.98, 154.29, 135.67, 123.47, and 122.01 nymphs and pupae plant-1 respectively.
Among the 25 castor genotypes screened, none of the genotypes were found absolutely free from the whitefly attack. Nine genotypes viz., ACH-2008, RG-2466, DPC- 9, DCH-177, 48-1, RG-34, RG-229, ACH-2146, and ACH-2020, showed resistant reaction with 1 score on a 0-5 scale. Seven genotypes viz., PCH-111, RG-380, DCS-9, Haritha, ACH-2033, RG-683, and DCS-107 were found moderately resistant with 2 score on a 0-5 scale. Six genotypes viz., GCH-8, RG-642, GCH-7, ICH- 66, GCH-4 and ICH-5 showed a moderately susceptible reaction with 3 score on a 0-5 scale. Three genotypes were viz., YRCH-1, M-574, and DCH-519, were found susceptible with 4 score on a 0-5 scale (Table 4.2).The results are in conformity with Haran et al. (2024), who reported that the YRCH-1 genotype was susceptible to whitefly with a score of 4 on a 0-5 scale, due to the presence of triple bloom on the leaves. The results obtained from this observation were also in accordance with Barad (2005), who reported that the triple bloom variety GCH-4 and double bloom variety DCS-47 were found highly susceptible to whitefly infestation. The double bloom genotypes viz., 48-1, DCS-9, GCH-5, DCS-89, DCS-33 and GCH-6 were found moderately susceptible to whitefly.
Bloom
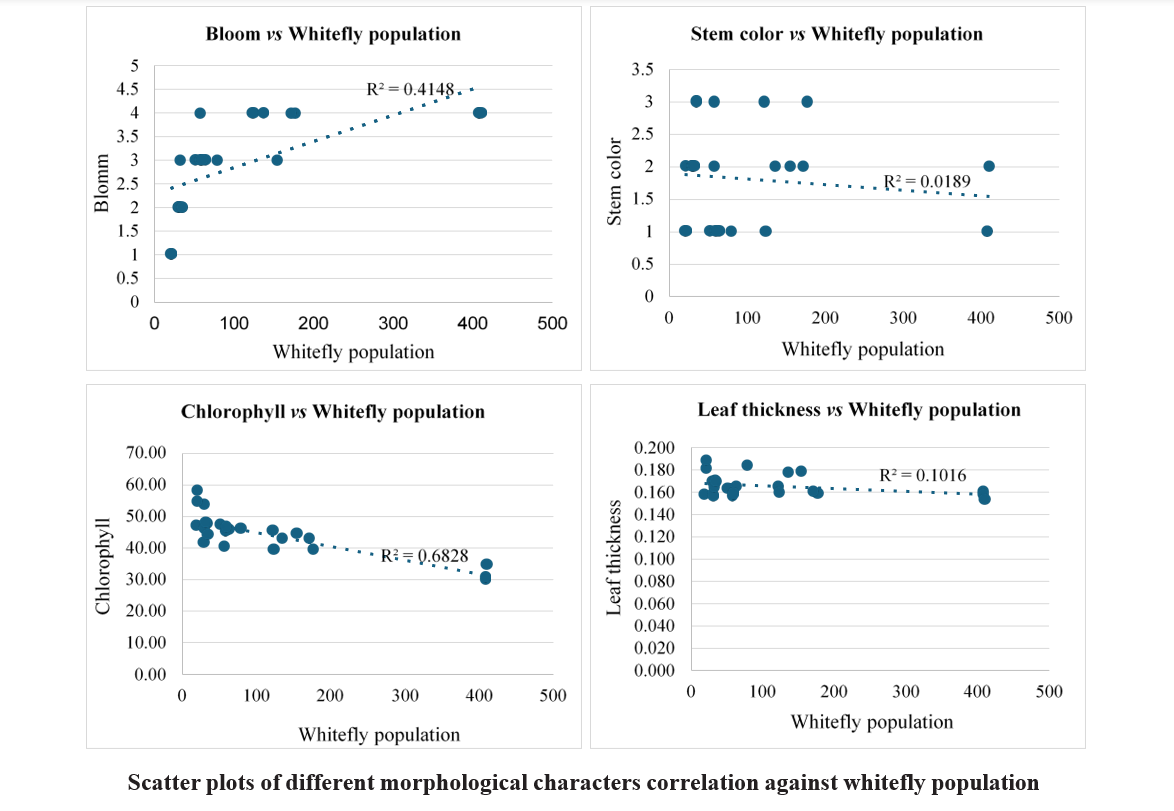
In the present investigation, four types bloom viz., zero, single, double and triple bloom were observed on different castor genotypes. The genotypes with zero bloom viz., ACH-2008, RG-2466 and genotypes with single bloom viz., ACH-2020, DCH-177 (Table 4.2) recorded comparatively low whitefly population and demonstrated a resistant reaction. In contrast, triple bloom genotypes such as YRCH-1, M-574 recorded the higher whitefly populations and were exhibited a susceptible reaction indicating a significant positive correlation (r= 0.644**) between bloom type and whitefly population (Table 4.3). It was in conformity with the findings of David and Paul (1973) who reported that the no-bloom castor varieties, along with the single-bloom varieties, were resistant to whitefly infestation. In contrast, the double and triple blooms, exhibited susceptibility to whitefly attacks.
Stem color and Leaf thickness
Three stem color types, namely Green, Red, and Mahogany, were observed among the different castor genotypes. Stem color did not showed any significant correlation with whitefly population (r= -0.137 NS) as most of the resistant genotypes recorded red stem color. On the other hand, the resistant check (DPC-9) has a green color stem. The correlation studies between the leaf thickness of different castor genotypes and whitefly population revealed a non-significant negative association (r= – 0.318 NS).
Chlorophyll content
The chlorophyll content expressed as SPAD Chlorophyll Meter Readings (SCMR). Among all the genotypes, RG-2466 recorded the highest (58.36) SPAD value, followed by ACH-2008 (54.86) and RG-34 (53.88) which were on par with each other. The lowest SCMR value was recorded on M-574 (30.18), and YRCH-1 (34.73). A significant negative correlation (r= -0.826**) was observed between chlorophyll content and whitefly population. From these observations, it was inferred that higher SCMR values in resistant genotypes represent dark green color leaves, while the lower SCMR values in susceptible genotypes denote light green to yellowish leaves, which are more attractive to whitefly adults for oviposition and feeding. These findings were in concurrence with that of Husain and Trehan (1940) who reported that whiteflies were more attracted to yellow- green color when compared to red, orange red, and dark green leaves. Further Devi et al. (2019), reported that female whiteflies show high color preferences to the castor leaves for oviposition.
CONCLUSION
Among the 25 castor genotypes screened against castor whitefly, nine genotypes showed resistant reaction, seven genotypes were found moderately resistant, six genotypes were showed a moderately susceptible reaction and three genotypes were found susceptible. The genotypes with zero and single bloom were found resistant, while the genotypes with triple bloom showed susceptible reaction. A significant negative correlation was observed between chlorophyll content and whitefly
population in different castor genotypes, whereas, non- significant negative correlation observed between stem color, leaf thickness and whitefly population.
LITERATURE CITED
Agyenim-Boateng, K.G., Lu, J.N., Shi, Y.Z and Yin, X.G. 2018. Review of leafhopper (Empoasca flavescens): A major pest in castor (Ricinus communis). Journal of Genetics and Genomic Sciences. 3: 009.
Barad, A.H. 2005. Population Dynamics, Varietal susceptibility and Chemical control of Castor Whitefly, Trialeurodes ricini (Misra). M.Sc. (Ag) Thesis. Junagadh Agricultural University, Junagadh, Gujarat, India.
Bink-Moenen, R.M., 1983. Revision of the African whiteflies (Aleyrodidae), mainly based on a collection from Tchad: 1-24.
David, B.V. and Paul, A.V. N. 1973. Studies on resistance of Castor to the whitefly, Trialeurodes rara Singh. Free amino acids. Madras agricultural Journal. 60(9-12): 1499-1503.
Devi, H.C., Kumari, P.K and Sobita, D.K. 2019. Morphological and phenotypic variability in blackgram genotypes with varying reaction to Mungbean Yellow Mosaic Virus (MYMV) infection. Journal of Pharmacognosy and Phytochemistry. 8(4):1606-1610.
FAO. Agriculture Production Database. Food and Agricultural Organization. 2020. Available from: https://www.fao.org .
Haran, H.R., Venkatachalam, S.R., Arutchenthil, P., Saravanan, P.A and Natarajan, S.K. 2024. Bloom and whitefly resistance in Castor bean (Ricinus communis L.): a genetic and linkage analysis. Genetic Resources and Crop Evolution. 172(3): 3683-3697.
Husain. M.A and Trehan, K.N. 1940. Final report on the scheme of investigations on the whitefly on cotton in the Punjab. Indian Journal of Agricultural Science. 10:101-109.
Idriss, M., Abdallah, N., Aref, N., Haridy, G and Madkour, 1997. Biotypes of the castor bean whitefly Trialeurodes ricini (Misra) (Hom., Aleyrodidae) in Egypt: biochemical characterization and efficiency of geminivirus transmission. Journal of Applied Entomology. 121: 501-509.
Ramanjaneyulu, A.V., Anudradha, G., Ramana, M.V., Reddy, A and Gopal, N.M. 2017. Multifarious uses of castor (Ricinus communis L.). International Journal of Economic Plants. 4(4):170-176.
Ranga, P., Singh, B., Guruwan, D., Yadav, S.S., Chauhan, A.S and Dahiya, P. 2022. Impact of sowing dates on incidence of major insect pests of castor (Ricinus communis L.) in South-Western Haryana. International Journal of Tropical Insect Science. 42(1):685-696.
Ranganath, T.R., Shivanna, B.K., Htjgar, A.Y., Hegde, J.N and Shashidara, K.C. 2021. Population dynamics of insect pests of castor. Indian Journal of Entomology. 83(2): 235-237.
Snehalatha, G., Radhika, P., Hariprasad, K., Kumar, V.D., Murugan, P.D. and Chalam, M.S.V. 2024. Survey on the incidence of sucking insect pests in rainfed castor. Andhra Pradesh Journal of Agricultural Sciences: 10(2): 132-136.
- Effect of Sowing Window on Nodulation, Yield and Post – Harvest Soil Nutrient Status Under Varied Crop Geometries in Short Duration Pigeonpea (Cajanus Cajan L.)
- Nanotechnology and Its Role in Seed Technology
- Challenges Faced by Agri Startups in Andhra Pradesh
- Constraints of Chcs as Perceived by Farmers in Kurnool District of Andhra Pradesh
- Growth, Yield Attributes and Yield of Fingermillet (Eleusine Coracana L. Gaertn.) as Influenced by Different Levels of Fertilizers and Liquid Biofertilizers
- Consumers’ Buying Behaviour Towards Organic Foods in Retail Outlets of Ananthapuramu City, Andhra Pradesh

