INTRODUCTION
Sugarcane is the most important commercial crop of India and plays a vital role in the agricultural as well as industrial economy. Sugarcane is a multipurpose crop that provides sugar, fiber, bio-fuel and manure apart from many by-products. It constitutes the major raw material for sugar production and for making gur and khandasari. Sugarcane has unique character of ratooning as several succeeding crops are raised from a single planting which is an integral component of the sugarcane production system.
Sugarcane (Saccharum officinarum) is a nutrient exhaustive crop that can uptake great amount of soil nutrients for its biomass production. In addition to micronutrient exportation, about 65 kg N, 90 kg P2O5 and 170 kg K2O are taken up for a target yield of 50 t ha-1 (Kathiresan, 2008). A permanent manurial trial, conducted for 33 years at RARS, Anakapalle (Andhra Pradesh), revealed that sugarcane crop without addition of fertilizers yielded about 40 t ha-1 of cane annually. The soil nitrogen reserve under this crop, however, increased by 50 per cent of the initial value which was clearly indicated that the root-associated diazotrophs contributed significant quantity of nitrogen for sustaining the production of sugarcane (Suman, 2003). Inoculation of N-fixing microbes to sugarcane has increased the cane yield by 5-15 per cent and also improved the juice quality parameters, viz., sucrose and purity (Hari, 1995).
Gluconacetobacter diazotrophicus is a nitrogen- fixing bacterium highly specific to sugar-rich crops. It can excrete about half of its fixed nitrogen in a form that plant can use. It has also been reported that besides N fixation, all the strains of G. diazotrophicus produced Indole acetic acid in a culture medium supplemented with tryptophan in the range of 0.14 to 2.42 l g ml-1 (Fuentez et al., 1993). Furthermore, it has been reported its ability to solubilize inorganic phosphates from the soil and make available P for the inoculated crops. Hence, Gluconacetobacter inoculation to sugarcane significantly increased the cane length, dry matter production and number of stalks, resulting in the more cane yield. PSB application which constitutes increased P solubilization which by production of organic acids which solubilizes the fixed form of phosphates into available form resulting in more available P in soil. KSB is more effective in releasing K from inorganic and insoluble fractions of total soil K through solubilization. With this view, a field experiment was conducted to study the effect of soil application and sett treatment of solid and liquid G. diazotrophicus, PSB and KSB along with fertilizers on growth characters and yield of sugarcane short crop.
MATERIAL AND METHODS
A field experiment was conducted during 2021-22 at Agricultural Research Station, Perumallapalle, Tirupati, Acharya N. G. Ranga Agricultural University, geographically situated at 13° 36′ 761” N latitude and 79° 20′ 704” E longitude with an altitude of 182.9 m above the mean sea level, which falls under Southern agroclimatic zone of Andhra Pradesh. The experiment soil was sandy loam in texture, neutral in reaction (7.36), normal in soluble salt concentration (0.232 dS m-1), low in organic carbon (0.49%), available nitrogen (212 kg ha-1) and medium in available phosphorus (40.12 kg ha-1) and high in available potassium (282 kg ha-1). The experiment consist of ten treatments viz., T1: 100% RDF, T2 : 125% RDF, T3: 100% RDF + soil application of solid Gluconacetobacter + PSB + KSB, T4 : 100% RDF + sett treatment with solid Gluconacetobacter + PSB + KSB, T5 : 75% RDF + soil application of solid Gluconacetobacter + PSB + KSB, T6 : 75% RDF + sett treatment with solid Gluconacetobacter + PSB + KSB, T7 : 100% RDF + soil application of liquid Gluconacetobacter + PSB + KSB, T8 : 100% RDF + sett treatment with liquid Gluconacetobacter + PSB + KSB, T9 : 75% RDF + soil application of liquid Gluconacetobacter + PSB + KSB and T10 : 75% RDF + sett treatment with liquid Gluconacetobacter + PSB + KSB was laid out in randomized block design with three replications.
The crop was sown with a seed rate of 40,000 three budded setts ha-1. The variety Swarnamukhi was planted. Recommended dose of inorganic fertilizers viz., 224:112:112 kg N : P2O5 and K2O ha-1, respectively were applied as per the treatments. Solid Gluconacetobacter, PSB and KSB were applied @ 10 kg ha-1 each as soil application. The recommended dose of solid biofertilizers for sett treatment was 10 kg – 1.25 kg – 1.25 kg ha-1 of Gluconacetobacter, PSB and KSB, respectively. Recommended dose of liquid Gluconacetobacter, PSB and KSB for soil application was 1 L, 1.25 L and 1.25 L ha-1, respectively. Similar quantity of liquid Gluconacetobacter, PSB and KSB was used for sett treatment. All the other recommended practices were also adopted as per the crop requirement. Data on dry matter production, tiller count, stalk population, cane length and cane yield was recorded at respective stages. The collected data was statistically analyzed by following the analysis of variance for randomized block design as outlined by
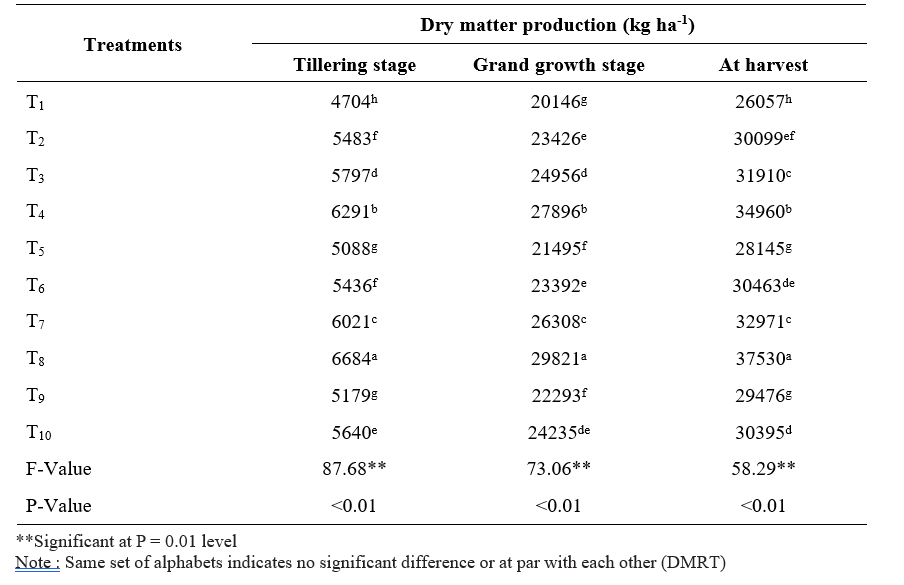
Table 1. Dry matter production of sugarcane short crop as influenced by application of microbial inoculants and inorganic fertilizers
Panse and Sukhatme (1985). Statistical significance was tested with ‘F’ test at 5 percent and 1 per cent level of probability. Further, multiple comparison tests have been done using Duncan’s multiple range test (DMRT) to identify the homogenous groups of treatments using SPSS-20.
RESULTS AND DISCUSSION
Dry matter production
Data pertaining to dry matter production at tillering, grand growth and harvest was represented in Table
- Application of 100% RDF + sett treatment with liquid Gluconacetobacter + PSB + KSB (T8) resulted significantly the highest dry matter production at all stages of crop growth (6684, 29821 and 37530 kg ha- 1, respectively) followed by 100% RDF + sett treatment with solid Gluconacetobacter + PSB + KSB (T4) at all stages of crop growth while the lowest was observed in control (100% RDF) (T1) (4704, 20146 and 26057 kg ha-1, respectively).
The highest dry matter production recorded with the combined application of 100% RDF and sett treatment with liquid Gluconacetobacter + PSB + KSB (T8). This might be due to higher germination per cent which leads to more tiller population, shoot population caused by bioinoculants which produces more growth promoting substances, increased availability of nutrients due to atmospheric nitrogen fixation in the rhizosphere, solubilization of mineral nutrients, nutrient recycling by microbial inoculants and also readily available nutrients from inorganic fertilizers. (Viana et al., 2019). These results are in line with the findings of Banerjee et al. (2018).
Tiller count and stalk population
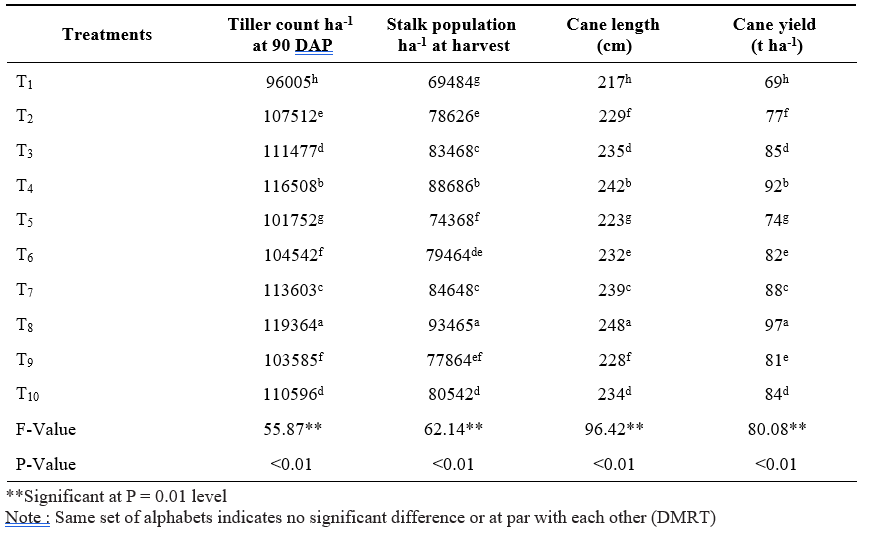
Data pertaining to tiller count and stalk population was presented in Table 2. Application of 100% RDF + sett treatment with liquid Gluconacetobacter + PSB + KSB (T8) resulted in significantly highest number of tillers at 90 DAP and stalk population at 240 days after planting followed by 100% RDF + sett treatment with solid Gluconacetobacter + PSB + KSB (T4). The lowest number of tillers and stalk population was observed in control (100% RDF) (T1). Improvement in plant population in terms of number of tillers and stalk population might be due to immediate supply of nutrients from inorganic fertilizer and sustained supply of nutrients from organics along with biofertilizers during the plant growth. Application of biofertilizers increased tiller number, and stalk population probably due to plant growth regulator hormones secreted by microbial inoculants. Ethylene is the foremost phytohormone regulating this physiological process in sugarcane (Mishra et al., 2014). Moreover, application of PSB has ability to produce cytokinins which will be essential for cell division in tiller buds. These results are in conformity with the findings of Thakur et al. (2010) and Singh et al. (2016).
Cane length
Cane length of sugarcane was significantly affected by the application of microbial inoculants along with fertilizers (Table 2). Significantly the highest cane length (248 cm) was observed with the application of 100% RDF + sett treatment with liquid Gluconacetobacter + PSB + KSB (T8) followed by 100% RDF + sett treatment with solid Gluconacetobacter + PSB + KSB (T4) (242 cm). The lowest cane length (217 cm) was observed with (100% RDF) (T1). Application of 100% RDF supplies nutrients in available form at initial stages of plant growth which plays an important role in metabolic process and activation of number of enzymes participating photosynthesis which inturn increased the plant growth and cane length. Application of biofertilizers improved soil environment in respect of nutrients for crop growth at active growing stages as a result of elevated root proliferation, cell multiplication and elongation leading to increased cane length. These findings are corroborated with the results obtained by Mathew and Varughese (2005), Shankaraiah (2007) and Singh et al. (2014).
Cane yield
Cane yield of sugarcane short crop was significantly differed with microbial inoculants and fertilizers application (Table 2). Significantly the highest cane yield (97 t ha-1) was recorded with the application of 100% RDF + sett treatment with liquid Gluconacetobacter
+ PSB + KSB (T8) and followed by 100% RDF + sett treatment with solid Gluconacetobacter + PSB + KSB (T4) (92 t ha-1). The control (100% RDF) (T1) produced significantly the lowest cane yield (69 t ha-1). The highest cane yield with 100% RDF + sett treatment with liquid Gluconacetobacter + PSB + KSB might be due to direct utilization of sugars present in setts by microbes as a food source which inturn leads to more microbial multiplication and leads to production of growth promoting substances. It helps in photosynthesis and translocation of substrates from source to sink i.e., cane and leads to more cane yield. Sufficient quantity of nutrients supplied through chemical fertilizers provides readily available nutrients and application of biofertilizers may hasten the constant nutrient supply by nitrogen fixation in the rhizosphere, solubilization of mineral nutrients, enhanced rooting and
Table 2. Tiller count, stalk population, cane length and cane yield of sugarcane short crop as influenced by application of microbial inoculants and inorganic fertilizers
plant establishment, better uptake of immobile nutrients such as P, improved nutrient cycling, improved plant tolerance to stress (biotic and abiotic) and amelioration of physical and biological environment. (Surendran and Vani, 2013). Similar results were reported by Indi et al. (2014) and Vajantha et al. (2019).
It can be concluded that combined application of 100% RDF + sett treatment with liquid Gluconacetobacter + PSB @ + KSB @ 1.25 is the most efficient nutrient management practice to obtain better growth, higher yields and quality of sugarcane short crop. Hence, it is the best practice to sustain higher productivity and to achieve economic profitability in Southern Agroclimatic Zone of Andhra Pradesh.
LITERATURE CITED
Banerjee, K., Puste, A.M., Gunri, S.K., Jana, K and Barman, M. 2018. Effect of integrated nutrient management on growth, yield, quality and soil health of spring planted sugarcane (Saccharam officinarum L.) in West Bengal. Indian Journal of Agronomy. 63(4): 41-47.
Fuentes, R.L.E., Jimenez, S.T., Abarca, O.I.R and Caballero, M.J. 1993: Acetobacter diazotrophicus, an indole acetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil. 154: 145-150.
Hari, K. 1995. Biofertilizers in sugarcane. Lead paper presented in 10th Sugarcane Research and Development Workers’ Meeting for South Karnataka, Shimoga, Karnataka, India.
Indi, D.V., Nalawade, S.V., Deshmukh, S.U and Pawar,
S.M. 2014. Response of sugarcane varieties to nitrogen and phosphorus as inoculated by Gluconacetobacter diazotrophicus and PSB. International Journal of Plant and Soil Science. 3(3): 260-269.
Kathiresan, G. 2008. Influence of organics and biofertilizers with graded levels of major nutrients on sugarcane yield, quality and economics under the soil having low and medium status of NPK. Cooperative Sugar. 39(10): 45-49.
Mathew, T and Varughese, K. 2005. Integrated nutrient management for sustainable cane production. Indian Journal of Agronomy. 50(3): 231-235.
Mishra, S., Nailwal, T.K and Pant, R.C. 2014. In vitro study of role of ethylene during tillering in sugarcane. Sugar Tech. 16: 255–263.
Panse, V.G and Sukhatme P.V. 1985. Statistical methods for Agricultural Workers, New Delhi.
Shankaraiah, C. 2007. Nitrogen management through biological process on nitrogen use efficiency in sugarcane and environmental protection. Sugar Tech. 9(2&3): 132-136.
Singh, D., Singh, V., Yadav, R.D and Singh, N. 2014b. Effect of organic and inorganic sources of nutrients on growth and productivity of sugarcane ratoon in sub-tropics. Cooperative Sugar. 45(9): 23-30.
Singh, S.C., Yadav, S.P., Yadav, S., Yadav, S.K., Tiwari, A.K and Sharma, B.L. 2016. Studies on plant geometry and nutrient management strategy in relation to mechanization in sugarcane (Saccharum spp. hybrid). Agrica. 5(2): 116-118.
Suman, A. 2003. Biological nitrogen fixation in relation to improving sugarcane productivity. In Summer School held at I.I.S.R., Lucknow. 15: 61-64.
Surendran, U and Vani, D. 2013. Influence of arbuscular mycorrhizal fungi in sugarcane productivity under semiarid tropical agro-ecosystem in India. International Journal of Plant Production. 7(2): 269-278.
Thakur, S.K., Jha, C.K., Kumari, G and Singh, V.P. 2010. Effect of Trichoderma inoculated trash, nitrogen level and biofertilizer on performance of sugarcane (Saccharum officinarum) in calcareous soils of Bihar. Indian Journal of Agronomy. 55(4): 308-311.
Vajantha, B., Sarala, N.V., Hemanth Kumar, M and Subba Rao, M. 2019. Effect of organic and inorganic fertilizers on available nutrient status, yield and joggery of sugarcane. International Journal of Current Microbiology and Applied Sciences. 8(9): 1456-1462.
Viana, R.S., Moreira, B.R.A., Lisboa, L.A.M., Junior, R.S., Nogueira, T.A.R., Figueiredo, P.A.M., Filho, M.C.M.T and Ramos, S.B. 2019. Morphological changes in sugarcane crop induced by the plant growth promoting bacterium Azospirillum brasilense. Sugar Tech. 1-9.