Evaluation of Different Methods of Artificial Inoculation Of Rhizoctonia Solani of Rice
0 Views
MALIKIREDDY SWATHI*, M.K. JYOSTHNA, R. SARADA JAYALAKSHMI DEVI, L. PRASANTHI AND S. GOPALAKRISHNAN
Department of Plant Pathology, S.V. Agricultural College, ANGRAU, Tirupati-517502.
ABSTRACT
Diseased samples of sheath blight was collected from the fields of RARS, Nandyal and isolation of pathogen was done by following different methods. Evaluation of different methods of artificial inoculation of R. solani was carried out. The efficiency of R. solani infection was tested on susceptible cultivar, NLR-34449 using four different methods (agar block, liquid cultured mycelia ball, mycelia suspension and typha bit method). Observations were taken 10 days after inoculation by measuring the relative lesion height (RLH), where RLH in each tiller was calculated. Typha bit inoculation method of R. solani longer lesions than other methods, liquid cultured mycelia balls, including agar block and mycelia suspension. Inoculation by Typha bit method was found to be more efficient for artificial inoculation of R. solani. The pathogen was re-isolated and maintained in PDA slants for further studies.
KEYWORDS: Isolation, Inoculation, Pathogenicity, Koch postulates.
INTRODUCTION
Rice (Oryza sativa) is the most important food crop in the world, feeding more people than any other crop. It is the staple food across Asia and is becoming increasingly important in Africa and Latin America. In India, rice is cultivated in an area of 44.5 M ha and 117.94 Mt of production with 2650 Kg ha-1(Govt. of India, Ministry of Agriculture, Department of Agriculture and Cooperation, Directorate of Economics & Statistics, 2020).
Low productivity of rice is due to several biotic and abiotic factors. It is very important to protect the crop from biotic factors. Rice is attacked by a number of fungal, bacterial and viral diseases. Among the fungal diseases of rice, rice sheath blight is regarded as an internationally important disease along with rice blast. Sheath blight is a soilborne disease caused by the fungus Rhizoctonia solani Kuhn AG1-IA (Anastamosis Group -IA). Pathogen survives in the soil as hard, weather resistant structure known as sclerotia which survive in the soil for more extended periods and tend to accumulate in the soil. This disease causes both qualitative and quantitative losses and yield losses usually range from 4-50 per cent depending on the crop stage at which the disease appears, extent of severity and the environmental condition. (Singh et al., 2004; Bhunkal et al., 2015).
Many different inoculum sources have been used for studying rice sheath blight like tooth picks colonized with R. solani (Rodrigues et al., 2003), colonized agar plugs (Eizenga et al., 2002), infected rice grain–rice hull mixtures (Kim and Rush 1986), blended mycelia (Sato et al., 2004), and sclerotia (Singh et al., 2001). Most of these inoculum sources introduce considerable variability into the infection process. Sclerotia, for example, vary widely with respect to their germination rate before infection based on their age and size (Singh et al., 2001). Each of these inoculum sources was used with its own inoculation method, under controlled conditions. As effective inoculation method is a critical component of an accurate disease assay for quantifying levels of sheath blight resistance among rice cultivars.
This study was undertaken to evaluate an effective, uniform, and reproducible technique for infecting rice plants with R. solani.
MATERIAL AND METHODS
Collection of Diseased samples
Diseased samples of sheath blight of rice were collected from Regional Agricultural Research Station (RARS), Nandyal. The samples were collected in brown paper bags and labelled with the information of date, place and name of the sample collected. The samples were bought to the laboratory for isolation of pathogen, Rhizoctonia solani.
Isolation of Rhizoctonia solani
Rhizoctonia solani causing sheath blight of rice was isolated from the rice plants showing typical sheath blight symptoms by tissue segment method (Rangaswami and Mahadevan, 1999).
Leaf sheath showing typical symptoms was washed in tap water for few minutes and leaf bits of 3-8 mm size were surface sterilized with 1% sodium hypochlorite for one minute and then rinsed thrice with sterile distilled water to remove the traces of sodium hypochlorite. The surface sterilized leaf bits were dried on two folds of sterilized filter blotting papers to remove excess moisture under aseptic conditions and were transferred aseptically to petri plates containing PDA and incubated at 28 ± 1°C. When the growth of fungus from leaf bits was seen on PDA surface, the hyphal bits from the periphery of culture growing in the petri plates was transferred to PDA in culture tubes. The culture thus purified by hyphal tip method and the culture was maintained in PDA slants.
Isolation of Rhizoctonia solani from sclerotial bodies
Rhizoctonia solani was also isolated from sclerotial bodies by placing sterile sclerotial bodies sterilized with 70% and washed thrice with sterile distilled water on a petri plate containing sterilized PDA medium. Plates were incubated at 28 ± 1°C and observed periodically for growth of the fungus. The culture was purified by single hyphal tip method and maintained on PDA by periodical transfer throughout the present investigation.
Identification of Rhizoctonia solani
- solani was identified based on its mycelial and sclerotial characters described by Lee and Rush (1983).
Evaluation of different artificial inoculation methods
One of the sheath blight susceptible varieties of rice, NLR-34449 (Nellore Mahsuri) was used in this study. To compare the effects of different artificial inoculation methods on the severity of sheath blight, four inoculation methods were used viz., (i) agar block (diameter 0.5 cm),
(ii) liquid cultured mycelia ball (diameter 0.5 cm), (iii) 100-µl mycelial suspension (105 CFU/ml) and typha bit method (4-5 bits per hill).
Agar block method
After placing R. solani mycelia or sclerotia onto potato dextrose agar (PDA) and grown at room temperature (22 to 24°C) under continuous light, agar blocks (0.5-cm squares) were cut and prepared from the outer edge of a 3-day-old culture.
Liquid cultured mycelia ball
Liquid cultures were obtained by inoculating 200 ml of potato dextrose broth. Rice plants at late tillering stage were inoculated with R. solani by placing a mycelial ball beneath the leaf sheath. The inoculated sheath was covered immediately with aluminum foil. When typical lesions appeared after 3 days, the aluminum foil was removed. R. solani infected plants were left in a humidity chamber made of clear plastic for 3 weeks to allow for disease development. Plants were grown at 28°C under 14-h days in both the greenhouse and the humidity chamber.
Mycelial suspension
Inoculations using the agar block (3-day cultures) and mycelial suspensions were the same as described for the mycelia ball. For the mycelial suspension, 100 µl of homogenized mycelium was applied for each inoculation using a pipette. R. solani isolate was inoculated at the maximum tillering stage. Seven days after inoculation, the lesion length on the sheath of the inoculated plants was measured. Three replications were used for each inoculation method in the study. The results were verified by repeating the experiment two times. The pathogen was re-isolated from artificially inoculated plants. Culture which was found similar to the original culture were maintained to carry out further studies.
Typha bit method
The culture of R. solani was grown for 10 days on autoclaved typha stem cutting (T. angustata; an aquatic weed) soaked with a solution containing 2% sucrose and 1% peptone. R. solani isolate was inoculated at the maximum tillering stage by placing 4-5 typha stem cuttings colonized with pathogen inside each hill The humidity was maintained between 80 and 100% in humidity chambers from the time of inoculation to disease evaluation.
Disease evaluation
To evaluate efficacy of artificial inoculation method, the lesion length and in each sheath of inoculated plants were recorded. The total lesion length was the sum of the lesion length of leaves in a culm. Severity of symptoms was observed by recording relative lesion height (RLH) following Vidhyasekaran et al. (1997) where RLH in each tiller was calculated as
Relative lesion height % =

RESULTS AND DISCUSSION
Collection of sheath blight infected rice plants.
The disease was characterized by large spots with irregular margins formed in bands resembling rattlesnake skin pattern. Symptoms were seen on leaf sheaths spreading from base, extending upto boot leaf sheath and also up to panicles in severe cases. Sclerotial bodies were formed on the infected portions of rice plant which were white and brown in colour indicating early and maturity stages of scleotial bodies.
Isolation of Rhizoctonia solani
Rhizoctonia solani was isolated from infected samples by tissue segment method (Rangaswami and Mahadevan, 1999).
Identification of R. solani
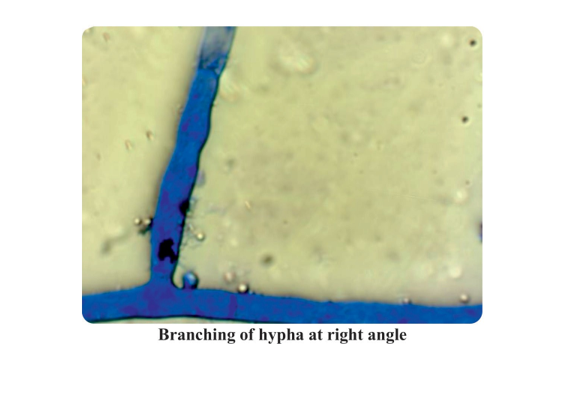
Cultural and morphological characters are important factors for identification of fungi. The cultural characteristics were recorded from the fully grown cultures in Petri plates. The culture obtained on PDA at 28±1oC was occupying the entire Petriplate in 3-4 days of incubation. The pathogen isolate produced irregular type of sclerotial bodies on PDA. While morphological characters were recorded by adapting slide culture technique under microscope. Microscopic examination of the fungal cultures revealed broad brown coloured hyphae branching at right angles. The culture of R. solani produced white mycelia characters. The size of scleotial bodies
Based on the morphological and colony characteristics described by Lee and Rush (1983) the causal organism was identified as R. solani.
Table 1. Comparison of four methods for rating the disease severity was made to determine which method produced the most accurate disease severity data among
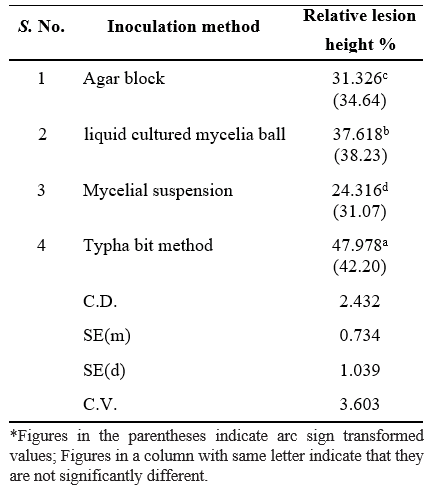
Evaluation of different artificial inoculation methods
Typha bit inoculation method produced the longest lesions on rice sheaths 7 days after inoculation. These results were significant and consistent across all replications tested (P < 0.001). Typha bits are hallow and have large surface area which helps in the penetration of R. solani into it and thus provide greater fungal biomass than the other inoculation methods. Mycelial suspensions were the least efficient inoculation method, possibly because the mycelium has been broken apart, preventing the allocation of nutrients through the mycelial network to the point of infection. Also, the mycelial suspensions were liquid and their retention at the site of inoculation varies depending on the architecture of the cultivar inoculated. The colonized agar block contained organized mycelia only on the top surface and does not have the same fungal biomass of the mycelial balls. Also, the nutrients provided by the agar block may have encouraged saprophytic growth in



the fungus rather than the development of an infection cushion needed for disease development. Rapid and uniform infection reduces the variation in the disease assays. In this study, infection rate was high with typha bit method. The isolate produced typical sheath blight symptoms on the susceptible variety NLR, 34449 that was characterized by large spots with irregular margins formed in bands resembling rattlesnake skin pattern. The pathogen was re-isolated from the diseased sample and it culture was similar to originally inoculated pathogen.
Artificial inoculation of R. solani by typha bit method was found more effective to develop an effective, uniform, and reproducible technique for infecting rice plants with R. solani.
ACKNOWLEDGEMENT
The first author is extremely thankful to Acharya N.G. Ranga Agricultural University, Guntur, A.P. for assisting us and conduct of experiment.
LITERATURE CITED
Bhaktavatsalam, G., Satyanarayana, K., Reddy, A.P.K and John, V.T. 1978. Evaluation for sheath blight resistance in rice. International Rice Research Newsletter. 3: 9-10.
Bhunkal, N., Singh, R and Mehta, N. 2015. Assessment of losses and identification of slow blighting genotypes against sheath blight of rice. Journal of Mycology and Plant Pathology. 45: 285-292.
Eizenga, G.C., Lee, F.N and Rutger, J.N. 2002. Screening Oryza species plants for rice sheath blight resistance. Plant Disease. 86: 808-812.
Kim, K.H and Rush, M.C. 1986. Inheritance of infection cushion formation by Rhizoctonia solani Kühn on rice leaf sheath. Korean Journal of Breeding. 18: 167-173.
Lee, F.N and Rush, M.C. 1983. Rice Sheath Blight: A major Rice Disease. Plant Disease. 67(7): 829-832.
Rangaswamy, G and Mahadevan, A. 1999. Diseases of Crop Plants in India. Prentice Hall of India. New Delhi. 607.
Rodrigues, F.Á., Vale, F.X.R., Datnoff, L.E., Prabhu, A.S and Korndörfer, G.H. 2003. Effect of rice growth stages and silicon on sheath blight development. Phytopathology. 93: 256-261.
Sato, H., Ideta, O., Ando, I., Kunihiro, Y., Hirabayashi, H., Iwano, M., Miyasaka, A., Nemoto, H and Imbe, Mapping QTLs for sheath blight resistance in the rice line WSS2. Breeding Science. 54: 265- 271.
Singh, A., Rohilla, R., Singh, U.S., Savary, S., Willocquet, L and Duveiller, E. 2001. An improved inoculation technique for sheath blight of rice caused by Rhizoctonia solani. Canadian Journal of Plant Pathology. 24:65-68.
Singh, S.K., Shukla, V., Singh, H and Sinha, A.P. 2004. Current status and impact of sheath blight in rice (Oryza sativa L.). Agricultural Reviews. 25 (4): 289-297.
Vidhyasekaran, P., Ruby Ponmalar, T., Samiyappan, R., Velazhahan, R., Vimala, R., Ramanathan, A., Paranidharan, V and Muthukrishnan, S. 1997. Host- Specific Toxin Production by Rhizoctonia solani, the Rice Sheath Blight Pathogen. Biochemistry and Cell Biology. The American Phytopathological Society. 87(12):1258-1263.
- Effect of Sowing Window on Nodulation, Yield and Post – Harvest Soil Nutrient Status Under Varied Crop Geometries in Short Duration Pigeonpea (Cajanus Cajan L.)
- Nanotechnology and Its Role in Seed Technology
- Challenges Faced by Agri Startups in Andhra Pradesh
- Constraints of Chcs as Perceived by Farmers in Kurnool District of Andhra Pradesh
- Growth, Yield Attributes and Yield of Fingermillet (Eleusine Coracana L. Gaertn.) as Influenced by Different Levels of Fertilizers and Liquid Biofertilizers
- Consumers’ Buying Behaviour Towards Organic Foods in Retail Outlets of Ananthapuramu City, Andhra Pradesh

