Bio-Formulations for Plant Growth-Promoting Streptomyces SP.
0 Views
V. SRINIVAS, S. PRATYUSHA AND S. GOPALAKRISHNAN*
International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru 502324, Telangana.
ABSTRACT
A total of 19 strains of Streptomyces sp. viz., CAI-13, CAI-17, CAI-21, CAI-24, CAI-26, CAI-68, CAI-78, CAI-85, CAI-
93, CAI-121, CAI-127, CAI-140, CAI-155, KAI-26, KAI-27, KAI-32, KAI-90, KAI-180 and MMA-32 were earlier reported by
us as plant growth-promoters in chickpea, pigeonpea, sorghum, rice, pearl millet, tomato and chili. In the present investigation, the 19 strains of Streptomyces sp. were evaluated for development of a long term, eco-friendly and inexpensive formulation using different carrier materials such as peat, lignite, talc and talc + starch. Streptomyces strains were individually grown and inoculated into the four selected carrier materials and stored in the refrigerator at 4oC. Samples were collected at the end of every month and tested for their viability and shelf life by plate count method for 12 months. All the 19 Streptomyces strains were viable and grown in all the tested carrier materials, however, peat inoculants showed greater viability and maintained stable colony forming units when compared talc, lignite, and talc + starch inoculants. It is concluded that peat formulation is best suited for mass multiplying the selected 19 Streptomyces strains..
KEYWORDS: Plant Growth Promoting (PGP) microbes, bio-formulations, Streptomyces sp.
INTRODUCTION
The use of beneficial microorganisms in agriculture has considerably increased over the last couple of decades due to the awareness of the environmental pollution due to synthetic pesticides and fertilizers, public health and sustainable agriculture (Mishra and Arora, 2016; Reigart and Roberts, 2013). Microorganisms and their products, including secondary metabolites, have been widely recognized as potential biocontrol as well as plant growth-promoting agents (Bashan et al., 2014). Actinomycetes such as Streptomyces represent an excellent alternative for improving nutrient availability to crop plants and promoting innovation and sustainability in agricultural systems (Vijayabharathi et al., 2018a, b). These attributes may be due to features such as quorum sensing controlled genes expression, multiplication rate, antibiotics, siderophores, cellulases, phytohormones, amino acid synthesis, chitinases, lipases, β-1, 3-glucanase production and ACC-deaminase production. Streptomyces and their secondary metabolites are widely reported to help the crops in enhancing shoot and root growth, nodulation and nitrogen fixation, grain yield, solubilisation of inorganic minerals (such as phosphorus, potassium and zinc) and biocontrol of insect pests and plant pathogens (Aggarwal et al., 2016; Bhattacharyya et al., 2017). Earlier, we reported 19 strains of Streptomyces having plant growth-promotion (PGP) potential in chickpea, pigeonpea, sorghum, rice, pearl millet, tomato and chili (Gopalakrishnan et al., 2011; 2012; 2013; 2014; 2015a, b, c; 2016a, b; 2020a; 2021; 2022; Pratyusha and Gopalakrishnan, 2023; Sathya et al. 2016; Srinivas et al., 2020, 2022).
In order to improve the potential of an efficient beneficial microbe under field conditions, it is important to optimize its mass multiplication protocols and formulations that enhances product quality, shelf-life and bioactivity. Selection of a good carrier material for the formulation is very critical as it can determine the failure or success of an bioagent application and disease management. The selection of a carrier material depends on how they support the beneficial microbes in good physiological conditions, shelf life, bioactivity, economic affordability and ease of application (Macik et al., 2020). Carrier materials that are widely reported to be used in formulation include charcoal, talc, cellulose powder, peat, farm yard manure (FYM), compost, lignite and press mud, whereas each formulation has its advantages and disadvantages (Gopalakrishnan et al. 2016c; Berninger et al., 2018). Generally, for Streptomyces, the formulations such as peat (a carbonized vegetable tissue formed in wet conditions by decomposition of various plants and mosses) and talcum (a natural mineral referred as steatite or soapstone and chemically referred as magnesium silicate, Mg3Si4O10 (OH)2; its very low moisture equilibrium enables for longer storage periods) are widely preferred (Bhattacharyya et al., 2020).
In the present study, the 19 strains of Streptomyces which were evaluated earlier under field conditions
on different crops, were tested for the development of a long term, eco-friendly and inexpensive formulations using different carrier materials such as peat, lignite, talc and talc + starch.
MATERIALS AND METHODS
Streptomyces strains used in this study
All 19 Streptomyces strains used in this study were isolated earlier from different herbal vermi-composts and rhizosphere soils and reported as PGP and biocontrol agents in crops including chickpea, pigeonpea, sorghum, rice, pearl millet, tomato, and chili (Gopalakrishnan et al., 2011; 2012; 2013; 2014; 2015a, b; 2016a, b; 2020a;
2021; Sathya et al. 2016; Srinivas et al., 2020). Of the 19 Streptomyces strains, the genome sequences of 16 Streptomyces strains, showing potential for PGP activities in various crops have been decoded (Gopalakrishnan et al., 2020b).
Viability and shelf life of different carrier bio-
formulations
A total of four carrier materials were tested that
includes peat, lignite, talc and talc + starch. The selected
19 strains of Streptomyces were inoculated (30 ml) individually on sterilized peat/lignite/talc/ talc + starch- based carrier materials (40 g) and allowed to multiply at room temperature (28±2 oC) for two-weeks. At the end two-week incubation, formulated peat/lignite/talc/talc
+ starch-based inoculants were evaluated for survival and longevity (shelf life) of Streptomyces, and this was considered as ‘0’ month. The survival and longevity of bio-inoculants was tested by plate count method. In brief, physiological saline tubes were prepared, and serial dilution of the formulations was done by mixing 10 g of inoculants in 90 ml saline, and serially diluted up to 10-8 dilutions. Samples (0.1 ml of 10-3-10-8dilutions) were spread plated on actinomycetes isolation agar followed by the incubation of plates at 27±1 oC for 96 h. At the end of incubation, the Streptomyces colonies were counted and represented as colony forming units (CFU) and the CFU was enumerated at one-month interval for a period of 12-months.
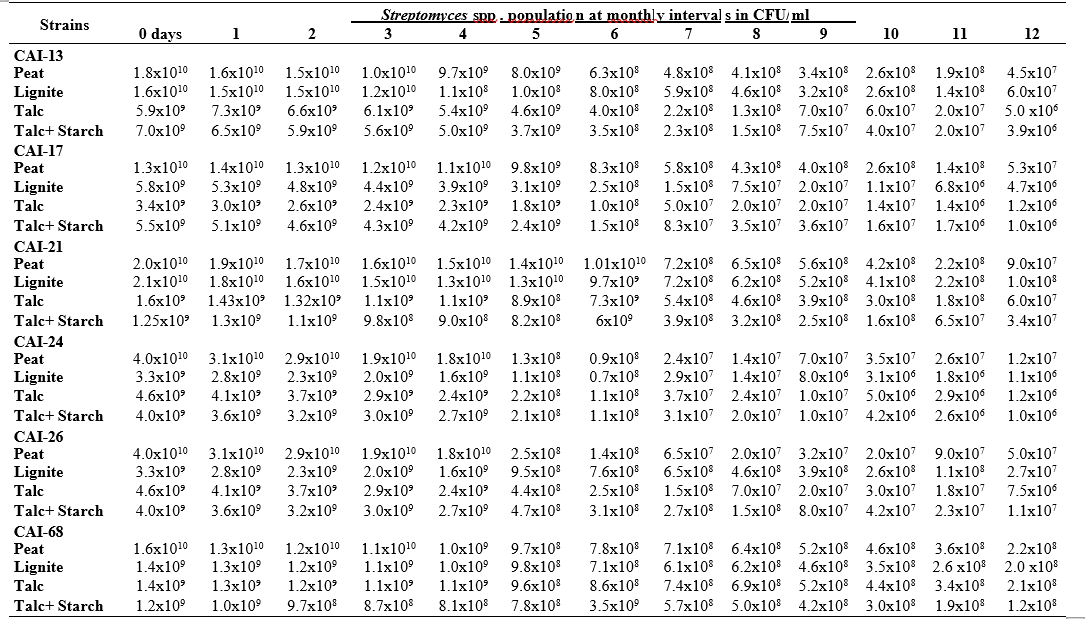
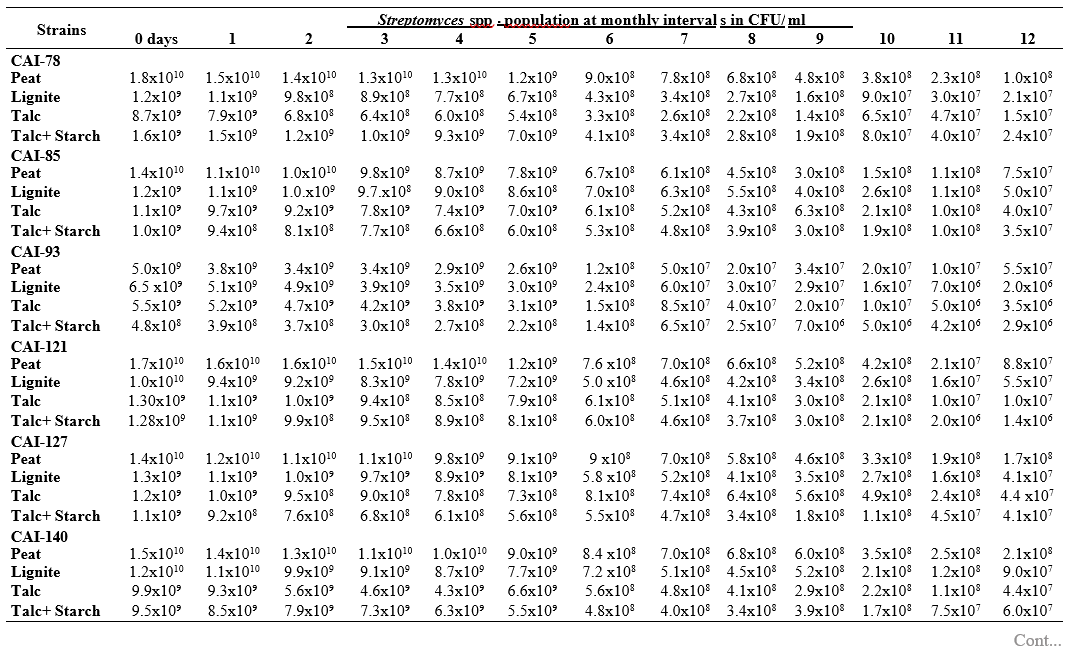
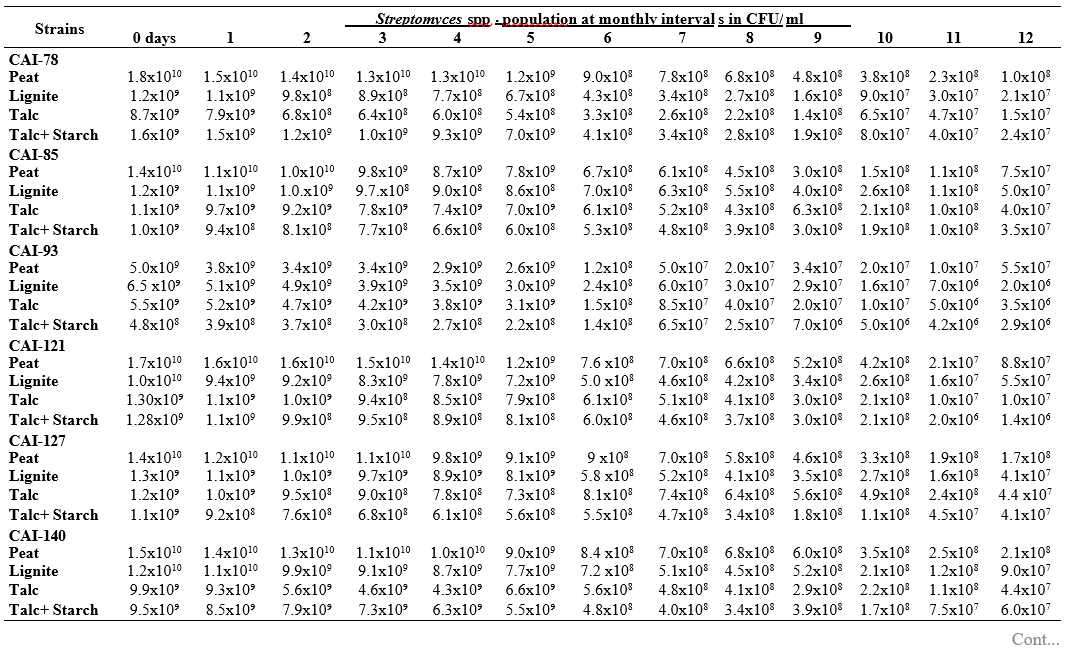
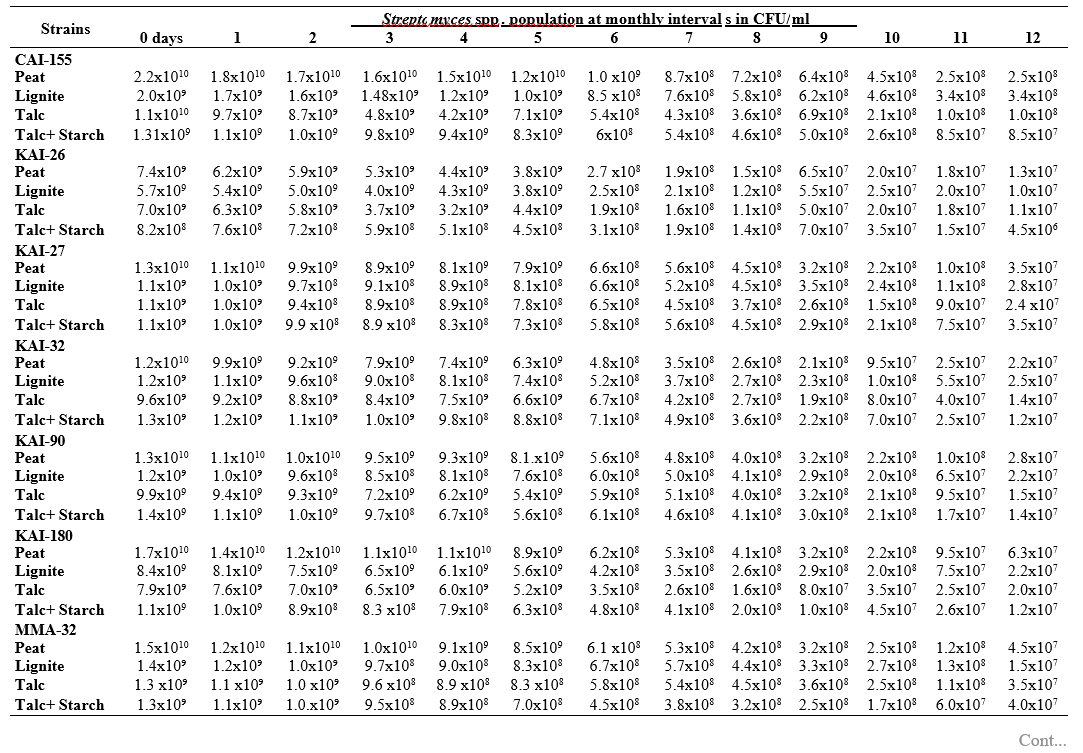
RESULTS AND DISCUSSION
To maximize the potential of the PGP microbe’s application in the field conditions, it is essential to stabilize them and increase their shelf-life (Bishnoi, 2015). In this regard, bio-formulations were made that includes an active microbe and a carrier material, which stabilizes and protects the microbial moiety (Aamir et al., 2020). Different types of carrier materials are available namely, charcoal, cellulose powder, lignite, peat, talc, vermiculite, and diatomaceous earth (Gopalakrishnan et al., 2016c; Zayed, 2016). In the present investigation, we evaluated four different carrier materials such as peat, lignite, talc and talc + starch for their ability to support our 19 Streptomyces strains which were demonstrated earlier as PGP and biocontrol agents under field conditions. The results showed that all 19 Streptomyces strains survived and maintained (at least 106 CFU/ ml) up to 12 months in all the four tested formulations. Of the four tested formulations, peat inoculants showed greater viability and maintained stable colony forming units when compared talc, lignite and talc + starch carriers. In the peat formulations, all the 19 peat inoculants survived and maintained at least 107 CFU/ ml up to 12 months while five peat inoculants (CAI-68, CAI-78, CAI-127, CAI-140 and CAI-155) were found to have at least 108 CFU/ ml up to 12 months. Next to the peat, lignite was also found to suit better for Streptomyces strains as 3 of the 19 strains (CAI-21, CAI-68 and CAI-155) maintained at least 108 CFU/ ml up to 12 months (Table 1). It is concluded that peat formulations were best suited for mass multiplying the selected 19 Streptomyces strains.
In the present study, all the four carrier bio- formulations were effective and showed promising viability of the 19 Streptomyces isolates up to a period of 12 months. This indicates the protective nature of the carrier materials and their ability to support the growth of the Streptomyces strains. Literature evidence also supports the usage of these carrier materials and mainly the peat formulations in particular, are known to evenly distribute and stabilize the microbes (Bashan et al., 2014; Bhattacharyya et al., 2020). Peat-based formulation is the widely used and most marketed PGP microbial inoculants and is most commonly used in bio-products producing industries (Gopalakrishnan et al., 2016c). In peat-based formulations, beneficial bacteria are metabolically very active and its multiplication continues during the storage and/or incubation periods as long as sufficient moisture, nutrients, and the optimum temperatures are maintained (Deaker et al. 2011). However, one of the main drawback of the peat formulations is its unavailability in many countries.
The survival and establishment of PGP microbe under field conditions in the rhizosphere in competition with native microbial flora is absolutely essential in order to avail the maximum benefits out of it. In the present study, it is evident that the peat and lignite carrier materials followed by talc and talc + starch are efficient in stabilizing and maintaining the viability of the selected 19 agriculturally important Streptomyces strains up to a year. This key information could be further explored for commercial production of the PGP Streptomyces strains
Table 1. Viability and longevity of 19 strains of Streptomyces spp. on four different formulations (Peat, lignite, talc and talc + starch) for 12 months
at a low cost and in a sustainable manner.
ACKNOWLEDGMENTS
This work has been undertaken as part of the CGIAR Research Program on Grain Legumes Dry Land Cereals.
LITERATURE CITED
Aamir, M., Rai, K.K., Zehra, A., Dubey, M. K., Kumar, S and Shukla, V.2020. “Microbial bioformulation- based plant biostimulants: a plausible approach toward next generation of sustainable agriculture,” in Microbial Endophytes Functional Biology and Applications, A. Kumar and E. K. Radhakrishnan (eds.; Oxford: Elsevier).195–225.
Aggarwal, N., Thind, S.K. and Sharma, S., 2016. Role of secondary metabolites of actinomycetes in crop protection. In: “Plant Growth-Promoting Actinobacteria”, Gopalakrishnan, S., Sathya, A., Vijayabharathi, R., (eds.), Springer Singapore, PP. 99─122.
Bashan, Y., de-Bashan, L.E., Prabhu, S.R and Hernandez JP. 2014. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998─2013). Plant Soil. 378:1–33
Berninger, T., González López, Ó., Bejarano, A., Preininger, C and Sessitsch, A. 2018. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microbial Biotechnology. 11: 277–301.
Bhattacharyya, C., Bakshi, U., Mallick, I., Mukherji, S., Bera, B and Ghosh, A. 2017. Genome-guided insights into the plant growth promotion capabilities of the physiologically versatile Bacillus aryabhattai strain AB211. Frontiers in Microbiology. 8: 411.
Bhattacharyya, C., Roy, R., Tribedi, P., Ghosh, A and Ghosh, A. 2020. “Bio-fertilizers as substitute to commercial agrochemicals,” in Agrochemicals Detection, Treatment and Remediation Pesticides and Chemical Fertilizers, M. N. V. Prasad (ed.; Elseveier). 263–290.
Bishnoi, U. 2015. PGPR interaction: an ecofriendly approach promoting the sustainable agriculture system. Advances in Botanical Research. 75: 81– 113.
Deaker, R., Kecskés, M.L., Rose, M.T., Amprayn, K., Ganisan, K., Tran, T.K.C., Vu, T.N., Phan, T.C., Hien, N.T and Kennedy, I.R. 2011. Practical methods for the quality control of inoculant bio-fertilisers. ACIAR Monograph Series No.147, Canberra, p 101
Gopalakrishnan, S., Kiran, B. K., Humayun, P., Vidya, S., Deepthi, K and Rupela, O. 2011. Biocontrol of charcoal-rot of sorghum by actinomycetes isolated from herbal vermi-compost. African Journal of Biotechnology. 10 (79):18142–18152.
Gopalakrishnan, S., Humayun, P., Srinivas, S., Vijayabharathi, R., Ratnakumari, B and Rupela, O. 2012. Plant growth- promoting traits of biocontrol potential Streptomyces isolated from herbal vermi-compost. Biocontrol Science & Technology. 22 (10):1199–1210.
Gopalakrishnan, S., Srinivas, V., Shravya, A., Prakash, B., Ratnakumari, B., Vijayabharathi, R and Rupela,
- 2013. Evaluation of Streptomyces spp. for their Plant growth-promoting traits in rice. Canadian Journal Microbiology. 59: 534–539.
Gopalakrishnan, S., Srinivas, V., Prakash, B., Arumugam, S., Vijayabharathi, R. and Rupela, O., Kudapa, H., Krishnamohan, K and Varshney, R. K. 2014. Evaluation of Streptomyces strains isolated from herbal vermi-compost for their plant growth- promotion traits in rice. Microbiological Research. 169:40–48.
Gopalakrishnan, S., Srinivas, V., Alekhya, G., Prakash, B., Kudapa, H., Rathore, A and Varshney,
R.K. 2015a. The extent of grain yield and plant growth enhancement by plant growth-promoting broad-spectrum Streptomyces sp. in chickpea. SpringerPlus. 4 (31):1–10.
Gopalakrishnan, S., Srinivas, V., Alekhya, G., Prakash, B., Kudapa, H and Varshney, R.K. 2015b. Evaluation of Broad-Spectrum Streptomyces sp. for Plant Growth Promotion Traits in Chickpea (Cicer arietinum L.). Philippine Agriculture Scientist. 98(3): 270–278.
Gopalakrishnan, S., Srinivas, V., Alekhya, G and Prakash, B. 2015c. Effect of plant growth-promoting Streptomyces sp. on growth promotion and grain yield in chickpea (Cicer arietinum L). 3 Biotech. 5: 799–806.
Gopalakrishnan, S., Sathya, A and Vijayabharathi, R. 2016a. A book entitled “Plant Growth-Promoting Actinobacteria: A New Avenue for Enhancing the Productivity & Soil Fertility of Grain Legumes” published by Springer Singapore ISBN 978-981- 10-0705-7.
Gopalakrishnan, S., Srinivas, V and Sameer Kumar, C.V. 2016b. Plant growth-promotion traits of Streptomyces sp. in pigeonpea. Legume Perspectives. 11: 43–44.
Gopalakrishnan, S., Sathya, A., Vijayabharathi, R and Srinivas, V. 2016c. Formulations of Plant Growth- Promoting Microbes for Field Applications. In: Microbial Inoculants in Sustainable Agricultural Productivity: Functional Applications. Springer India, India, pp. 239-251.
Gopalakrishnan, S., Sharma, R., Srinivas, V., Naresh, N., Mishra, S.P., Ankati, S., Pratyusha, S., Govindaraj, M., Gonzalez, S.V., Nervik, S and Simic, N. 2020a. Identification and characterization of a Streptomyces albus strain and its secondary metabolite organophosphate against charcoal rot of sorghum. Plants. 9:1727.
Gopalakrishnan, S., Thakur, V., Saxena, R.K., Vadlamudi, S., Purohit, S., Kumar, V., Rathore, A., Chitikineni, A and Varshney, R.K. 2020b. Complete genome sequence of sixteen plant growth promoting Streptomyces strains. Scientific Report. 10(1):10294.
Gopalakrishnan, S., Srinivas, V., Naresh, N., Mishra, S.P., Ankati, S., Pratyusha, S., Madhuprakash, J., Govindaraj, M and Sharma, R. 2021. Deciphering the antagonistic effect of Streptomyces spp. and host-plant resistance induction against charcoal rot of sorghum. Planta. 253:57.
Gopalakrishnan, S., Srinivas, V., Chand, U., Pratyusha, S and Samineni, S. 2022. Streptomyces consortia- mediated plant growth-promotion and yield performance in chickpea. 3Biotech. 12:318.
Macik, M., Gryta, A and Frac, M. 2020. Bio-fertilizers in agriculture: an overview on concepts, strategies and effects on soil microorganisms. Advances in Agronomy. 162: 31–87.
Mishra, J and Arora, N.K. 2016. Bio-formulations for plant growth promotion and combating phytopathogens: a sustainable approach, in Bio- formulations: for Sustainable Agriculture, N. K. Arora, S. Mehnaz, and R. Balestrini (eds. New Delhi: Springer). 3–33.
Pratyusha, S and Gopalakrishnan, S. 2023. Streptomyces– mediated synthesis of silver nanoparticles for enhanced growth, yield and grain nutrients in chickpea. Biocatalysts and Agricultural Biotechnology. 47: 102567.
Reigart, J and Roberts, J. (2013). Recognition and Management of Pesticide Poisonings. Washington, DC: Office of Pesticide Programs, U.S. Environmental Protection Agency. 272.
Sathya, A., Vijayabharathi, R., Kumari, B.R. et al. 2016. Assessment of a diketopiperazine, cyclo(Trp- Phe) from Streptomyces griseoplanus SAI-
25 against cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Applied Entomology and Zoology. 51:11–20.
Srinivas, V., Sravani, A., Pratyusha, S and Gopalakrishnan, S. 2021. Amazing Plant Growth-Promoting Actinobacteria from Herbal Vermicompost. Andhra Pradesh Journal of Agricultural. Sciences. 7: pp. 89-98.
Srinivas, V., Naresh, Pratyusha, S., Ankati, S., Govindaraj M and Gopalakrishnan, S. 2022. Enhancing pearl millet hybrids performance for yield and nutrients through plant growth-promoting Streptomyces spp.: An agronomic biofortification approach. Crop & Pasture Science 73(5):484-493.
Vijayabharathi, R and Gopalakrishnan, S., Sathya, A., Srinivas, V and Sharma, M. 2018a. Deciphering the tri-dimensional effect of endophytic Streptomyces sp. on chickpea for plant growth promotion, helper effect with Mesorhizobium ciceri and host-plant resistance induction against Botrytis cinerea. Microbial Pathogenesis (TSI). 122: 98-107.
Vijayabharathi, R and Gopalakrishnan, S., Sathya, A., Vasanth Kumar, M., Srinivas, V and Mamta, S. 2018b. Streptomyces sp. as plant growth-promoters and host-plant resistance inducers against Botrytis cinerea in chickpea. Biocontrol Science and Technology (TSI). 1-24.
Zayed, M.S. 2016. “Advances in formulation development technologies,” in Microbial Inoculants in Sustainable Agricultural Productivity, eds. D.Singh, H. B. Singh, and R. Prabha (New Delhi: Springer). 219–237.
- Effect of Sowing Window on Nodulation, Yield and Post – Harvest Soil Nutrient Status Under Varied Crop Geometries in Short Duration Pigeonpea (Cajanus Cajan L.)
- Nanotechnology and Its Role in Seed Technology
- Challenges Faced by Agri Startups in Andhra Pradesh
- Constraints of Chcs as Perceived by Farmers in Kurnool District of Andhra Pradesh
- Growth, Yield Attributes and Yield of Fingermillet (Eleusine Coracana L. Gaertn.) as Influenced by Different Levels of Fertilizers and Liquid Biofertilizers
- Consumers’ Buying Behaviour Towards Organic Foods in Retail Outlets of Ananthapuramu City, Andhra Pradesh

